Dear Editor,
Field therapy is a popular and important treatment for actinic keratosis. These agents have unique benefits as well as side effects. Dermatologists and others prescribing topical treatment for actinic keratosis should be aware of several important side effects. One of the most important side effects is the development of impetigo on the treated skin. We present a case of a patient who developed impetigo after applying ingenol mebutate gel for treatment of actinic keratosis. Impetigo is a not uncommon, but important complication of topical therapy for actinic keratosis. Prompt diagnosis and treatment can prevent unnecessary morbidity.
Topical pharmacotherapy for treatment of actinic keratosis (AK) is an alternative preferred by some dermatologists over lesion-directed modalities because the former allows for coverage of subclinical AKs (so called “field treatment”). There are several options for dermatologists and patients considering field treatment with a topical agent. Traditionally 5-fluorouracil (5-FU) has been used, but this requires consistent daily to twice daily application for up to four weeks. In comparison, ingenol mebutate (IM) requires only once daily applications for two or three days. Such a schedule allows for enhanced patient adherence. Furthermore, a recent prospective randomized trial confirmed that the local skin reaction (LSR) of patients treated with IM is more short lived compared to those treated with 5-FU (1).
Topical pharmacotherapy agents are generally well tolerated. Nearly all patients receiving topical pharmacotherapy for AKs notice erythema and discomfort at the site of treatment. In a multicenter, randomized, double-blind study, pain and pruritus were found to be the most commonly reported adverse events with IM use (2).
A 70-year-old Caucasian male with prior non-melanoma skin cancer history presented with three erythematous macules with adherent scale on the lip and forehead. These were clinically determined to be AKs and were treated with combination cryotherapy and field treatment of the face with IM 0.015% gel for three days. The patient returned four weeks later with bright erythema and honey-colored crust of the upper and lower lip (Figure 1). A bacterial culture was collected and at that time the patient was treated empirically with mupirocin and cephalexin. The bacterial culture subsequently revealed pan-sensitive staphylococcus aureus and the patient rapidly improved.
Erythema of the Upper and Lower Cutaneous and Mucosal Lip With Honey-Colored Crusting
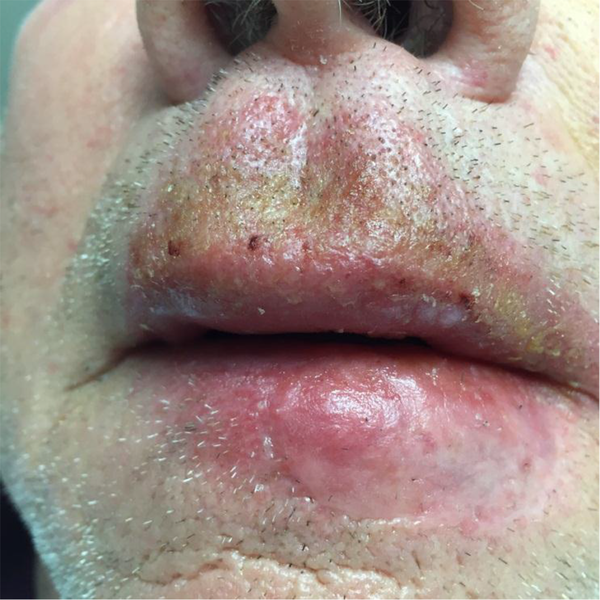
Impetigo during treatment with 5-FU for AK is not uncommon and is presumably a result of a compromised skin barrier. Reasonably, the incidence of impetigo could be expected to be higher in patients receiving 5-FU compared to IM as the LSR is more persistent in the former (1). In a study of 162 patients receiving IM, only one patient developed impetigo (3). Prompt recognition and treatment of this condition is essential to prevent unnecessary morbidity. While IM has a preferable treatment schedule and duration of LSR, it nevertheless can result in the adverse effects like impetigo that are assumed to be more characteristic of 5-FU.
References
-
1.
Samorano LP, Torezan LA, Sanches JA. Evaluation of the tolerability and safety of a 0.015% ingenol mebutate gel compared to 5% 5-fluorouracil cream for the treatment of facial actinic keratosis: a prospective randomized trial. J Eur Acad Dermatol Venereol. 2015;29(9):1822-7. [PubMed ID: 25727104]. https://doi.org/10.1111/jdv.13063.
-
2.
Lebwohl M, Swanson N, Anderson LL, Melgaard A, Xu Z, Berman B. Ingenol mebutate gel for actinic keratosis. N Engl J Med. 2012;366(11):1010-9. [PubMed ID: 22417254]. https://doi.org/10.1056/NEJMoa1111170.
-
3.
Anderson L, Schmieder GJ, Werschler WP, Tschen EH, Ling MR, Stough DB, et al. Randomized, double-blind, double-dummy, vehicle-controlled study of ingenol mebutate gel 0.025% and 0.05% for actinic keratosis. J Am Acad Dermatol. 2009;60(6):934-43. [PubMed ID: 19467365]. https://doi.org/10.1016/j.jaad.2009.01.008.