Abstract
Background:
Human skin gas is known as traces of gas emanating from the human skin. Exogenous chemicals such as toluene may be released from the skin surface when absorbed into the body. However, dermal emission of toluene has not been fully determined in relation to inhalation exposure.Objectives:
This study aimed to characterise the mechanism of toluene emanating from the skin surface of healthy volunteers in relation to inhalation exposure.Methods:
Dermal emission flux of toluene was determined in healthy volunteers employing a passive flux sampler and gas chromatography-mass spectrometry.Results:
The dermal emission of toluene occurs when toluene is absorbed by inhalation. The half-life obtained from the decrease in the dermal emission flux suggested the time-course of the dermal emission corresponds to that of the blood concentration. The whole-body emission rate of toluene was estimated to be 9.9% of the uptake rate by inhalation exposure. This suggests the toluene is excreted approximately 80% through urine, 7% - 14% by exhaled air, and 10% from the skin surface.Conclusions:
This demonstrates that dermal emission is a newly discovered route of excretion of toluene from the human body and that dermal toluene might indicate the individual biological susceptibility to inhalation exposure to toluene.Keywords
1. Background
Human skin gas is known as traces of gas emanating from the human skin. It has been attracting considerable attention in relation to various roles such as a mosquito attractant (1), specific odour of elderly people (2), indicator of smoking (3) and drinking alcohol (4), non-invasive medical biomarkers for severe burns (5), acute poisoning (6), liver disease (7), and diabetes (8). Volatile compounds are formed by the internal metabolism and carried into the blood, and they can move to the skin surface with sweating and/or directly travel from the blood through the dermal layers as a vascular network lies beneath the skin (4). Exogenous volatile compounds absorbed into the body are also released via this route (3, 6). Therefore, the human skin is a possible excretion route of volatile compounds from the human body.
Toluene (IUPAC systematic name: methylbenzene) is commonly used as a solvent and is released from various products found in homes, including paints, adhesives, automotive products, and some personal care products (9). Exposure to toluene is known to cause adverse health effects such as headaches, dizziness, feelings of intoxication, eye, nose and throat irritation by laboratory and workplace studies (9). Hisanaga et al. (10) investigated the amount of chemicals emanating from the skin surface of 10 subjects and found an increased dermal emission of toluene from one male subject due to toluene exposure at his workplace. Sekine et al. (3) detected the dermal emission of toluene from both smokers and non-smokers due to unintentional passive smoking in indoor environments. However, dermal emission of toluene has not been quantitatively determined in relation to exposure in indoor environments.
2. Objectives
This study aimed to characterize the mechanism of toluene emanating from the skin surface of healthy volunteers and to identify the influence of being exposed to toluene in an indoor environment on the dermal emission employing a passive flux sampler (PFS) coupled and gas chromatography-mass spectrometry (GC-MS) (2, 3, 6). The PFS is a simple device developed by the authors to determine the dermal emission flux (emission rate per area) of volatile organic compounds (VOCs) (2, 3, 6). The sampler can be used anywhere non-invasively and be operated by non-medical professionals. In this study, volunteer tests were conducted to link the dermal emission and toluene inhalation in an indoor environment.
3. Methods
3.1. Human Skin Gas Analysis
Figure 1 shows a schematic representation of the PFS when it is applied to the skin surface. The device is composed of a glass vial with a polypropylene screw cap, disk type trapping medium (MonoTrap® DCC18, GL Sciences, Japan), and O-ring as a stopper. The PFS is first put on the skin surface to create a headspace with a height of 0.80 cm. VOCs emitted from the skin diffuse towards the trapping medium within the headspace and are collected on the medium. The DCC18 disk is made of silica embedded with activated carbon powders (11). Prior to its use, the open end of the sampler was capped with the glass vial and sealed with a piece of ParafilmTM. The sampler was then enclosed in an aluminium bag.
Schematic view of the passive flux sampler for the collection of toluene emanating from the skin surface

After sampling, the trapping medium was transferred into a glass vial and capped. Using a headspace sampler (STRAP, JEOL, Japan), the trapped VOCs were thermally desorbed at 120°C and were injected into the GC-MS system (JMS-Q1000GC MkII, JEOL, Japan) using a split ratio of 10 to 1. The VOCs were separated using the InertCap® Pure-Wax column (30 m × 0.25 mm I.D. × 0.25-µm film thickness; GL Sciences, Japan) by employing the following temperature program: 50°C hold for 2 min, increase at 15°C min-1 to 150°C hold for 5 min, increase at 25°C min-1 to 210°C hold for 5 min, and increase at 35°C min-1 to 250°C hold for 10 min. Helium (G1 grade, Taiyo Nippon Sanso, Japan) was used as the carrier gas at a flow rate of 1.0 mL.min-1. Peak responses on the samples were acquired using real time SIM: m/z 91 for toluene. The detector interface temperature was maintained at 250°C.
Following previous studies (2, 4, 6), the emission flux of toluene at a sampling position, E (ng cm-2 h-1), was calculated using the following formula:
where W is the amount (ng) of toluene collected, S is the effective cross-section of the trapping medium (0.594 cm2), and t is the sampling time (1.0 h). Procedure blanks were subtracted from the sample.
Excellent linearity was found between the concentration of toluene in methanol (0.10, 1.0, 2.0, 5.0, and 10 µg.mL-1), which corresponds to 2.6, 26, 1.7 × 102, 4.2 × 102, 8.4 × 102 ng cm-2 h-1 of the emission flux, and its corresponding peak area with r = 1.0. The recovery rate of the trapped toluene was investigated by administrating 0.5 µg of toluene vapour to the DCC18 disk and resulted in 100 % (n = 3). As significant contamination of toluene was detected in the trapping medium obtained from the commercial source, the medium was thermally pre-treated to reduce the blank level. The limit of quantification (LOQ) was defined as 10-fold standard deviation of multiple blanks (n = 5) and obtained from Equation 1. The LOQ of dermal emission flux of toluene resulted in 2.0 ng cm-2 h-1.
3.2. Volunteer Tests
Test 1: To investigate the effect of inhalation exposure of toluene in an indoor environment on the dermal emission, 10 healthy volunteers, 8 men (age: 21 - 24 years) and 2 women (age: 23 - 24 years), were recruited and divided into two groups A and B (n = 5 for each group). Before sampling, volunteers washed their left forearm using soap and tap water. After applying PFS to the skin surface of the left forearm, the forearm was covered with a sleeve to prevent surface contamination by toluene deposition in the air. During the first session, group A wore a single particulate respirator type N95 embedded with charcoal for trapping VOCs (SH9550C, NIOSH approved, San-Huei United), entered into a chemical laboratory where the toluene concentration was approximately 1.5 mg.m-3 (cf. threshold limit value for toluene is 20 ppm (12), 76.5 mg.m-3 at 293 K), and stayed at rest in the laboratory. Group B also stayed in the same room without wearing the N95 mask. At the second session, Group B wore the N95 mask and stayed at rest in the laboratory for 1 h and group A stayed without the mask in the same laboratory. All volunteers conducted human skin gas samplings for 1.0 h at each session. The room temperature was 293 K, and relative humidity was around 50%.
Test 2: To investigate variations in the dermal emission flux of toluene after exposure, four healthy volunteers, 3 men (age: 21 - 24 years) and 1 woman (age: 23 years), were recruited. The PFS was applied to the left forearm of participants. The participants stayed at rest in a general school class room for 1 h, before being moved into the chemical laboratory without a N95 mask and stayed at rest for 1 h, and then returned to the class room for 2 h. Human skin gas sampling was conducted before, during, and after exposure to toluene at every 1 h.
Test 3: To investigate the relationship between personal exposure concentration and dermal emission flux, 12 volunteers, 7 men (age: 21 - 24 years) and 5 women (age: 21 - 23 years), were recruited. The PFS was put on the left forearm of participants. Simultaneously, a passive air sampler (Passive Gas Tube, Sibata, Japan) was applied on the shirt collar to measure the personal exposure concentration of toluene. All participants stayed at rest in the chemical laboratory for 8 h. Following the sampling instruction of the passive air sampler, sampling duration was set at 8 h. Collection amount of toluene (µg) by the passive air sampler was determined using GC-MS and converted to air concentration using a sampling rate of 10.8 µg ppm-1.h-1 and sampling duration of 8 h. The room temperature was 293 K, and relative humidity was around 50%.
This study was performed in accordance with the principles of the Declaration of Helsinki and was approved by the Institutional Review Board, Shonan Campus, Tokai University, Japan (no.: 16181). Written informed consent was obtained from all study participants.
4. Results and Discussion
The dose-dependent relationship between the concentration of inhaled toluene and that found in the blood is well documented in humans (13, 14). The major metabolic pathway of toluene in the blood is the formation of benzyl alcohol and further oxidation to benzaldehyde and benzoic acid. Thereafter, benzoic acid is conjugated with glycine to form hippuric acid, and most of the toluene absorbed are excreted in the urine in the form of hippuric acid (15). Exhalation is also known as another excretion route of toluene in the blood (16). As it is a volatile and hydrophilic compound, toluene in the blood moves directly to the lung and is excreted with exhaled air. Meanwhile, our findings suggest that dermal emission is also a possible route of excretion of toluene from the blood.
Figure 2 shows the dermal emission flux of toluene of healthy volunteers with and without N95 mask. The dermal emission flux without wearing N95 mask ranged from < LOD to 1.1 × 102 ng cm-2 h-1 with an average of 47 ± 43 ng cm-2 h-1 (n = 9, one outlier was excluded). Meanwhile, those with N95 mask decreased to < LOD to 7.2 ng cm-2 h-1 with an average of 1.3 ±2.6 ng cm-2 h-1 (n = 9). This indicates that the dermal emission flux of toluene depends on the amount of toluene inhaled.
Comparison of the emission flux of toluene emanating from healthy volunteers at rest with and without wearing the N95 mask during exposure in the chemical laboratory for 1 hour. Bars show standard deviation of dermal emission fluxes (sampling site: forearm, indoor toluene concentration: 1.5 mg.m-3, n = 9, one outlier was excluded, Wilcoxon signed-rank test: P = 0.005).

Figure 3 shows the time-course of the dermal emission flux of toluene before and after inhalation. The dermal emission flux of toluene increased during exposure in the chemical laboratory and then decreased to the initial levels after moving to the school class room. The elimination kinetic of VOCs in a body is often described using the sum of two or three exponential expressions (17-19). According to Lof et al. (14), the elimination of toluene from the blood in humans is considered triphasic; the half-lives (t1/2) were 3 min for the initial very rapid elimination phase, 40 min for the rapid phase, and 738 min for the slow phase. Though the time-resolution was only for 1 h due to a limitation of analytical sensitivity in this study, the t1/2 obtained from the decrease in the dermal emission flux of four volunteers resulted in 46 minutes, which is in accordance with that of the rapid elimination phase in the blood reported by Lof et al. (13). This suggests the time-course of the dermal emission of toluene corresponds to that of the blood concentration.
Time-course of the dermal emission flux of toluene before and after the inhalation of 1.5 mg.m-3 of toluene. Bars show standard deviation of dermal emission fluxes (n = 4, sampling site: forearm).

Monitoring of the personal exposure concentration of VOCs using a passive air sampler has been accepted as an alternative to that of the indoor concentration of VOCs in bulk air for occupational safety. Therefore, personal exposure was measured for 12 volunteers in the chemical laboratory and compared with the dermal emission flux of toluene. The personal exposure concentration ranged from 0.28 to 0.37 mg.m-3 (n = 12), whilst the dermal emission flux showed a greater variation from below LOQ to 82 ng cm-2 h-1 (n = 12) during the stay in the chemical laboratory for 8 h. There was no significant correlation (r = 0.14) between exposure concentration and the dermal emission flux of toluene due to greater variation in the individual dermal emission.
To investigate a mass balance between uptake and dermal elimination, the uptake rate by inhalation exposure and whole-body emission rate of toluene were roughly estimated. The uptake rate of toluene by inhalation exposure, U (mg.h-1), was calculated using Euation 2 as follows:
Where C is the individual personal exposure concentration of toluene (mg.m-3) and Q is the daily inhalation rate (17.3 m3.d-1) for Japanese people (20). Carlsson (16) reported that approximately 55% of inspired air was absorbed in the body of men exposed to 300 mg m-3 for 2 h at rest; this value dropped to 50% during the next 2 h of exposure at rest. Lof et al. (21) reported a similar absorption percentage (approximately 50% absorbed) in groups of 10 men exposed to approximately 300 mg m-3 at rest for 4 h. Then, the coefficient of 0.5 was used in this estimation.
In contrast, the whole-body dermal emission rate of toluene, D (mg.h-1), was calculated using Equation 3 as follows:
Where E is the individual dermal emission flux of toluene (ng cm-2 h-1) and S is the surface area of the forearm region of young Japanese (men: 895 cm2, women: 768 cm2). As the partial emission rate from the forearm region contributed to 20% of the whole-body emission rate of toluene (22), the ES was divided by 0.2 to obtain the whole-body dermal emission rate.
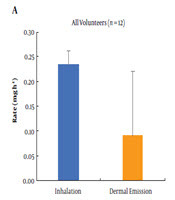
Figure 4 shows the comparison of inhalation rate and whole-body dermal emission rate of toluene for all volunteers (a) and selected non-smokers (b). The average whole-body dermal emission rate resulted in approximately 77% of the inhalation rate (D/U = 0.77) for all volunteers. According to previous studies, around 80% of the absorbed toluene is excreted in the urine (14) and between 7% and 14% is eliminated by exhalation (15). Considering these percentages, the 77% elimination via the skin surface is not in accordance with findings of those studies. Profiles of the participating volunteers revealed that four volunteers had a smoking habit. As cigarettes contain many VOCs, including toluene, cigarette smoking can be a source of dermal emission of toluene (3). Although smoking was prohibited during the experiment, the daily smoking habit probably increased the baseline toluene concentration in the blood and ultimately increased the dermal emission. After excluding the four smokers, the whole-body dermal emission reduced to 9.9% of the inhalation rate (D/U = 0.099). This emission would be in accordance with the previous studies, making the total excretion amount of the absorbed toluene roughly 80% in the urine, 7% - 14% in exhaled air, and 10% from the skin surface. However, there were some limitations in the estimation, especially due to uncertainty in the use of the respiration rate, skin surface area, and small number of participants.
Comparison between the estimated uptake and whole-body dermal emission rates of toluene in (A) all healthy volunteers and (B) non-smokers. Bars show standard deviation of rates.

The uptake of chemicals in environment occurs via three main routes, ingestion, inhalation, and dermal absorption, that lead to an internal concentration or body burden of the chemicals (23). Therefore, risk assessment of hazardous chemicals on human health is usually conducted based on the integrated uptake amount by the three routes. However, this study showed that toluene, whose uptake route is dominantly by inhalation, is released from the skin surface. This may also be the case when the dominant uptake route is by ingestion. The body internal concentration of the chemical is determined by the way of exposure, and physiological characteristics of individual. Therefore, the dermal emission of toluene may indicate the individual biological susceptibility to inhalation exposure to toluene.
In this study, the dermal emission flux of toluene was determined for healthy volunteers using the PFS coupled with GC/MS in relation to inhalation exposure in the chemical laboratory. The results showed that the dermal emission of toluene occurs when toluene is absorbed by inhalation. Therefore, dermal emission can be the “third” elimination route of absorbed toluene in addition to urinary excretion and exhalation. There was a great variation in the dermal emission flux of toluene among individuals, even when the study volunteers stayed in the same exposure environment. This suggests the dermal emission of toluene reflects physiological characteristics of individuals.
References
-
1.
Bernier UR, Kline DL, Barnard DR, Schreck CE, Yost RA. Analysis of human skin emanations by gas chromatography/mass spectrometry. 2. Identification of volatile compounds that are candidate attractants for the yellow fever mosquito (Aedes aegypti). Anal Chem. 2000;72(4):747-56. [PubMed ID: 10701259]. https://doi.org/10.1021/ac990963k.
-
2.
Kimura K, Sekine Y, Furukawa S, Takahashi M, Oikawa D. Measurement of 2-nonenal and diacetyl emanating from human skin surface employing passive flux sampler-GCMS system. J Chromatogr B Analyt Technol Biomed Life Sci. 2016;1028:181-5. [PubMed ID: 27362995]. https://doi.org/10.1016/j.jchromb.2016.06.021.
-
3.
Sekine Y, Sato S, Kimura K, Sato H, Nakai S, Yanagisawa Y. Detection of tobacco smoke emanating from human skin surface of smokers employing passive flux sampler - GCMS system. J Chromatogr B Analyt Technol Biomed Life Sci. 2018;1092:394-401. [PubMed ID: 29945104]. https://doi.org/10.1016/j.jchromb.2018.06.038.
-
4.
Takahashi K, Sekine Y, Furukawa H, Asai S, Miyachi H. Influence of acetaldehyde emanating from human skin on indoor air concentration level. Indoor Environment. 2013;16(1):15-22. https://doi.org/10.7879/siej.16.15.
-
5.
Kimura K, Sekine Y, Umezawa K, Furukawa S, Takahashi M, Asai S, et al. Clinical application of ammonia emanating from severe burn patients during critical car. J Jpn Assoc Odor Environ. 2016;47(6):421-9.
-
6.
Umezawa K, Sekine Y, Kimura K, Asai S, Saito T, Inokuchi S, et al. Emanation of fenitrothion from the skin surface of a patient who attempted to commit suicide by acute poisoning. Off J Jpn Soc Lab Med. 2018;66(9):949-56.
-
7.
Sekine Y, Kimura K, Umezawa K. What does human skin gas analysis work for? J Jpn Assoc Odor Environ. 2017;48(6):410-7.
-
8.
Yokokawa T, Sato T, Suzuki S, Oikawa M, Yoshihisa A, Kobayashi A, et al. Feasibility of skin acetone analysis in patients with cardiovascular diseases. Fukushima J Med Sci. 2018;64(2):60-3. [PubMed ID: 30012937]. [PubMed Central ID: PMC6141450]. https://doi.org/10.5387/fms.2018-03.
-
9.
Government of Canada. Toluene in indoor air. 2019, [cited 2019 Nov 5]. Available from: https://www.canada.ca/en/health-canada/services/environmental-workplace-health/reports-publications/air-quality/toluene-indoor-air-environment-workplace-health.html.
-
10.
Hisanaga M, Tsuda T, Ohkuwa T, Ito H. Determination of volatile organic compounds in human skin gas by GC/MS. Bunseki Kagaku. 2012;61(1):57-61.
-
11.
GL Sciences Inc. Monolithic silica adsorbents. 2019, [cited 2019 Sep 29]. Available from: http://www.glsciences.com/c-product/flavor-and-smell/monolithic-silica-adsorbents/.
-
12.
American Conference of Governmental Industrial Hygienists. Cincinnati, OH, USA. ACGIH:; 2011.
-
13.
Lof A, Wigaeus Hjelm E, Colmsjo A, Lundmark BO, Norstrom A, Sato A. Toxicokinetics of toluene and urinary excretion of hippuric acid after human exposure to 2H8-toluene. Br J Ind Med. 1993;50(1):55-9. [PubMed ID: 8431392]. [PubMed Central ID: PMC1061234]. https://doi.org/10.1136/oem.50.1.55.
-
14.
Astrand I; Modeling of inhalation exposure to vapors: Uptake distribution and elimination. Effect of physical exercise on uptake, distribution and elimination of vapors in man. 2. Florida, USA: CRC Press; 1983.
-
15.
Antti-Poika M, Kalliokoski P, Hanninen O. Toluene. In: Snyder R, editor. Ethel Browning's toxicity and metabolism of industrial solvents. 1. Amsterdam, Netherland: Elsevier; 1987. p. 38-63.
-
16.
Carlsson A. Exposure to toluene: Uptake, distribution and elimination in man. Scand J Work Environ Health. 1982;8(1):43-55. [PubMed ID: 7134922]. https://doi.org/10.5271/sjweh.2497.
-
17.
Scheepers PT, Konings J, Demirel G, Gaga EO, Anzion R, Peer PG, et al. Determination of exposure to benzene, toluene and xylenes in Turkish primary school children by analysis of breath and by environmental passive sampling. Sci Total Environ. 2010;408(20):4863-70. [PubMed ID: 20619876]. https://doi.org/10.1016/j.scitotenv.2010.06.037.
-
18.
Brugnone F, Perbellini L, Apostoli P, Locatelli M, Mariotto P. Decline of blood and alveolar toluene concentration following two accidental human poisonings. Int Arch Occup Environ Health. 1983;53(2):157-65. [PubMed ID: 6654512]. https://doi.org/10.1007/bf00378428.
-
19.
Benoit FM, Davidson WR, Lovett AM, Nacson S, Ngo A. Breath analysis by API/MS--human exposure to volatile organic solvents. Int Arch Occup Environ Health. 1985;55(2):113-20. [PubMed ID: 3988355]. https://doi.org/10.1007/bf00378373.
-
20.
National Institute of Advanced Industrial Science and Technology (AIST). Research Center for Chemical Risk Management. 2019, [cited 2019 Sep 29]. Available from: https://unit.aist.go.jp/riss/crm/exposurefactors/documents/factor/body/breathing_rate.pdf.
-
21.
Lof A, Wallen M, Wigaeus Hjelm E. Influence of paracetamol and acetylsalicylic acid on the toxicokinetics of toluene. Pharmacol Toxicol. 1990;66(2):138-41. [PubMed ID: 2315264]. https://doi.org/10.1111/j.1600-0773.1990.tb00720.x.
-
22.
Sekine Y, Kimura K, Sato S. Relationship between human skin gas and body odour. In Case studies on odour measurement and deodorization technology. Tokyo, Japan: Technical Information Institute; 2018. p. 18-27.
-
23.
Esteban M, Castano A. Non-invasive matrices in human biomonitoring: a review. Environ Int. 2009;35(2):438-49. [PubMed ID: 18951632]. https://doi.org/10.1016/j.envint.2008.09.003.