Abstract
Context:
The prevalence of non-alcoholic fatty liver disease (NAFLD) is about 30% in general population. Since conditions such as obesity, hyperlipidemia, diabetes mellitus, metabolic syndrome and insulin resistance have been named as some of the most important risk factors, as the prevalence of these conditions continues to rise, NAFLD becomes an increasingly significant problem. Endothelial dysfunction, increased pulse wave velocity, increased coronary artery calcification, and the development of atherosclerosis appear to be influenced by NAFLD. Considering this concern, this narrative review investigated the prevalence of CVD in NAFLD patients.Evidence Acquisition:
This narrative study evaluates the prevalence of CVD in NAFLD patients, as well as several variables that influence their association. In this study our search strategy engines include PubMed, Scopus, MEDLINE, and Google Scholar.Results:
Previous research suggests that as nonalcoholic steatohepatitis (NASH) and liver fibrosis progress, there is an increased risk of CVD, most likely due to the hepato-cardiovascular axis' effect. The correlation between NAFLD and metabolic disorders suggests that fatty liver disease may cause cardiovascular disorders (CVD).Conclusions:
As a result, the management of both NAFLD and the risk of CVD are aimed at the same target, which is to reduce insulin tolerance by lifestyle modifications. Moreover, monitoring for CVD and proper pharmacotherapy is essential in individuals with severe NAFLD and those who are at increased risk for ischemic heart disease.Keywords
Ischemic Heart Disease Nonalcoholic Fatty Liver Disease Coronary
1. Context
Non-alcoholic fatty liver disease (NAFLD) is a chronic condition which refers to the most common chronic liver diseases, with an incidence rate of 30 percent worldwide (1, 2). For example, in Europe, the median prevalence of NAFLD is ranged about 25 - 26% with wide variations in different populations. In Asia it is possible to observe, on average, a prevalence between 15 - 20% with a variable distribution among different countries. However, considering high risk population groups, in these areas NAFLD prevalence grows exponentially, 75 - 92% in overweight subjects and 60-70% in diabetic ones. On the other hand, as the prevalence of morbid obesity and diabetes mellitus continues to rise, NAFLD becomes an increasingly significant problem. In fact, about one-third of the population consists of overweight individuals. Obesity has also been associated with an increase in the occurrence of type 2 diabetes over the previous decade (3). Overall, conditions such as obesity, hyperlipidemia, diabetes mellitus, metabolic syndrome and insulin resistance have been named as some of the most important risk factors for NAFLD (4, 5). On the other hand, past studies have demonstrated that NAFLD includes different types of liver injury such as benign steatosis without hepatocellular injury, liver fibrosis and cirrhosis, which might sometimes progress to hepatocellular carcinoma, and nonalcoholic steatohepatitis (NASH) (6, 7). In 5 - 20% of patients with NAFLD, we witness a progression to NASH (8). Evidence suggests that inflammatory cytokines such as TNFα and IL-1β, some of which are produced by different T-cell populations, may play a significant role in NASH immune-pathogenesis (9).
Fatty liver disease is frequently detected when tests are conducted for other reasons, such as abnormal ultrasonography or a liver enzyme test, as it normally presents no symptoms. However, a person with NAFLD may have normal liver enzymes. The health care provider will make a diagnosis based on medical history, symptoms, a physical exam and blood tests. An ultrasound or other imaging studies may be performed. In some cases, the provider may order a test to measure the level of fibrosis in the liver, such as a liver biopsy or one of the newer noninvasive tests (10).
Reports from numerous studies on the correlation between NAFLD and metabolic disorders have suggested NAFLD might cause cardiovascular disorders (CVD). Patients with NAFLD have a higher mortality rate due to CVD, rather than as a result of liver disease itself (11). Therefore, fundamental and clinical research have been carried out to shed light on NAFLD as an independent risk factor for subclinical and clinical CVD (12). Endothelial dysfunction, increased pulse wave velocity, increased coronary artery calcification, and the development of atherosclerosis appear to be influenced by NAFLD (13). These results may stem from the effects of this disease on the hepato-cardiovascular axis (14) (Figure 1). The independent association between NAFLD and CVD may consist or vanish after the various risk factors for ischemic heart disease (IHD) such as obesity, hypertension, or diabetes are controlled (15).
Hepato-cardiovascular axis
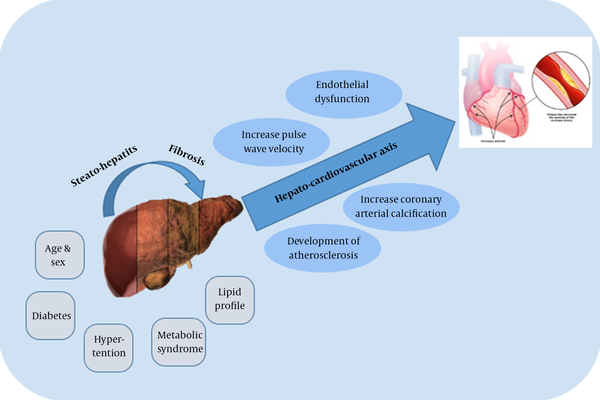
2. Evidence Acquisition
This narrative study evaluates the occurrence of CVD in NAFLD patients, and several variables that influence their association. In this study our search strategy engines include PubMed, Scopus, MEDLINE, and Google Scholar. Our search term includes “non-alcoholic fatty liver disease”, “cardiovascular diseases”, “metabolic disorders”, “non-alcoholic fatty liver disease AND cardiovascular diseases”, and “non-alcoholic fatty liver disease AND metabolic disorders”.
3. Results
3.1. Severity of NAFLD and CVD Risk
The risk of CAD in men and women 40 years of age is 49% and 32% respectively, throughout life. It has been estimated that the lifetime risk for individuals with 70 years old is about 35 percent and 24 percent in men and women respectively (16). The incidence of myocardial infarction (MI) or sudden death in men and women aged 65 to 94 is approximately two and three times higher than in people aged 35 to 64, respectively (17). Although in developed countries death due to CAD has been decreasing, the condition is different worldwide. Regardless of the country's affluence, CAD is the leading cause of mortality among adults (18).
There are many documents which have supported the relation between NAFLD and the increasing prevalence of CVD (19-21). Several factors can interfere with the results of studies on NAFLD. These include the baseline BMI, abdominal obesity, glucose level, diet including total calories, saturated fat, carbohydrates, choline and foods of varying glucose index, physical activity, use of drugs for diabetes, dyslipidemia and alcohol consumption (22).
Compared to the general population, patients with NAFLD have poor endothelial function (this was analyzed by conducting FMV, a test which could evaluate the vasodilator response of an artery in ischemic conditions) with a P value of less than 0.0001 (23). In addition, past investigation estimated that individuals with a 10-year of cardiovascular events might have moderately higher risk of NAFLD especially in patients with non-alcoholic steatohepatitis (23). Also, Motamed et al. found in a cohort study on 2804 individuals aged 40 - 74 years that according to two risk assessment tools (American College of Cardiology/American Heart Association and Framingham general cardiovascular risk profile) subjects with NAFLD were more likely to develop CVD within ten years (12). According to recent research, an injured endothelial monolayer might be partially repaired by circulating endothelial progenitor cells (EPCs) produced by the bone marrow (24). In a study of 312 consecutive patients with suspected coronary artery disease who underwent coronary artery angiography, 34 patients were diagnosed with NAFLD using abdominal ultrasonography (25). Their circulating EPC number and function was assessed, showing that those patients with NAFLD had a significantly reduced circulating EPC levels (P < 0.05) and decreased EPC function (25). According to Yilmaz et al., the development of NAFLD to liver fibrosis can be an independent determinant of reduced coronary flow reserve, implying a link between NAFLD severity and CVD risk (26).
A 5-year prospective cohort study of 1221 Japanese men and women, 231 of whom were diagnosed with NAFLD, found that NAFLD might be employed as a novel CVD predictor factor independent of other traditional risk factors (odds ratio 4.12, 95% CI, 1.58 to 10.75, P = 0.004) (27). Another research found that 58.2 percent of 612 patients had fatty liver using ultrasonography and 76.0 percent had coronary artery disease (28). Fatty liver was shown to be independently linked to coronary artery disease after adjustment of other factors including demographic and metabolic factors with an odd ratio (OR) of 2.31 and 95% CI of 1.46 to 3.64. However, it should be noted that the morbidity and mortality could not be predicted by fatty liver in patients with established coronary artery disease (28). Finally, Wang et al. discovered that the degree of fatty liver on ultrasonography can predict CVD risk in 874 individuals who had a routine health examination (29).
A systematic review and meta-analysis conducted by Wu et al., reported that NAFLD is not associated with overall mortality or CVD mortality (15). However, it is correlated with a higher risk of ischemic heart disease (HR = 2.31, 95% CI: 1.46 - 3.65) and a higher risk of (HR = 1.16, 95% CI: 1.06 - 1.27) hypertension (15). In 2016, a total of 15,913 individuals were enrolled in a study to estimate the 10-year risk of CVD severity in NAFLD patients (30). It has been estimated that 57.6% of individuals had no evidence of NAFLD, and 35.4%, 6.5% and 0.5% of them had grade I, grade II, and grade III of NAFLD, respectively. Additionally, results showed that the 10-year risk for CVD, was 1.52, 2.56, 3.35 for NAFLD grades I, grade II, and III respectively, after adjustment for conventional CVD risk factors, with a P value of less than 0.01 in comparison to the no-NAFLD group as a control. Therefore, experts suggest that simple steatosis is not correlated with increased mortality due to CVD or liver failure by itself. In contrast, patient mortality is associated with more progressed stages of NAFLD, and in particular with liver fibrosis (31). Although, there is some debate on the role of NAFLD in the CVD (32). Alexander et al. studied a large matched cohort study and found that NAFLD is not associated with CVD in 17.7 million population of the study (32).
Deaths from liver disease and cardiovascular disease are associated with higher mortality in those with NAFLD. The optimum treatment for NAFLD should alleviate liver problems while simultaneously lowering the risk of cardiovascular disease. However, this is an ambitious goal. Yet, in patients with NAFLD, a fasting lipid profile, including the small density LDL and HDL subtype, carotid intimal thickness, and indicators of systemic and vascular inflammation, such as c reactive protein, should be assessed (33).
3.2. Parameters Affecting Association of NAFLD and CVD
3.2.1. Age and Sex
CVD still remains the leading cause of death worldwide (34). Previous studies showed that CVD incidence and mortality are three and five times higher in men compared to women, respectively (35). In both men and women, increasing age increases the risk of CVD incidence and mortality, although the effect is greater in women (35). On the other hand, the reported difference in the prevalence of NAFLD in the two sexes has been variable. Earlier studies showed that NAFLD is more prevalent in women, however other studies suggest that this prevalence is the same in male and female populations (36). Furthermore, studies that described the correlation between NAFLD and increased risk of CVD often omitted the impacts of sex and age in order to prevent any confounding errors. Consequently, further research needs to be conducted to evaluate age and sex as independent risk factors for NAFLD and CVD.
3.2.2. NAFLD, Diabetes and CVD Risk
Metabolic factors are the cornerstone of NAFLD physiopathology. As mentioned above, the tight relation of NAFLD to metabolic syndrome could hypothesize the key role of NAFLD in increment of CVD risk (37). Diabetes mellitus is the main metabolic disorder, which can lead to NAFLD or affect its progress. Targher et al., conducted a study on 250 patients with type 1 diabetes, 44.4% of which had NAFLD (38). Results indicated that individuals diagnosed with NAFLD have a higher risk of coronary artery disease with a P value of less than 0.001 when age and sex balanced (10.8% vs. 1.1%, respectively) (38). In another study conducted by the same authors two years later, the previous fact was proved. Moreover, it was described that in patients with Type 1 diabetes, NAFLD is correlated with a higher prevalence of CVD. This is independent from several previously established risk factors such as the components of metabolic syndrome (39). It also described that, with a rate of 26.6%, NAFLD causes a higher prevalence of coronary artery disease among individuals with type 2 diabetes, by a P value of less than 0.001 (40). Another study was conducted on 2,103 type 2 diabetic individuals with no evidence of CVD at the study base line (38). During a 5-year follow-up, 248 individuals (11.8%) had ischemic stroke, nonfatal coronary heart disease, or died as a result of cardiovascular events. The prevalence of cardiovascular events estimated higher in those participants with NAFLD, after adjustment of factors including age, sex, diabetes duration, smoking history, LDL cholesterol, HbA1c, liver enzymes, and medications use (OR 1.84, 95% CI 1.4 - 2.1). In addition, after adjusting for metabolic syndrome, they observed decreasing in this association, but did not abolish it (OR 1.53, 95% CI 1.1 - 1.7) (38). Other research have revealed that hepatic fat content might be used as an independent predictor of myocardial insulin resistance and lower coronary functional capacity in patients with type 2 diabetes (41). This data describes a possible role for NAFLD in the pathogenesis of CVD.
3.2.3. NAFLD and Hypertension
Similar to NAFLD, hypertension is suggested to be associated with metabolic syndrome and as previously mentioned, both are risk factors for CVD (42). The relationship between NAFLD and CVD with carotid artery intima-media thickness (IMT) was studied in a study of 642 adolescents aged 11 to 13 years old (43). In this population, results showed that two independent markers which increased IMT included NAFLD and systolic blood pressure. Lau et al. conducted a study on 3191 individuals aged 20-79 years with a 5-year follow-up (44). It was suggested that individuals with fatty liver disease and elevated serum alanine transferase (ALT) levels have three times higher risk of hypertension at beginning and follow-up in comparison to individuals with no fatty liver disease (OR 2.8 and 3.1 respectively). One year later, López-Suárez et al. investigated the strength of the relation between NAFLD and hypertension regardless of ALT levels (45). Results confirmed that NAFLD is tightly correlated to an independent risk of identifying hypertension and high-normal systolic blood pressure, suggesting that NAFLD can be a risk factor for hypertension and further CVD. Further studies also showed that NAFLD is correlated to 24-h (P < 0.005), during day (P < 0.02) and night (P < 0.005) systolic blood pressure measurements but not with non-dipping hypertension (P = 0.057) (42).
3.2.4. Metabolic Syndrome and NAFLD
Metabolic syndrome is a set of clinical and biochemical abnormalities such as atherogenic dyslipidemia, abdominal obesity, insulin resistance, increased blood pressure, proinflammatory state, and prothrombotic state (46). CVD is the primary clinical outcome of metabolic syndrome. Additionally, in patients diagnosed with metabolic syndrome there is an increment of risk for type 2 diabetes, and diabetes itself is another main cause for CVD (47). Marchesini et al. conducted a study on 304 patients diagnosed for NAFLD (5). Results showed that 35 - 75% of the patients met the diagnostic criteria and 90% had more than one component of metabolic syndrome. It has been established that the frequency of metabolic syndrome in patients with NAFLD is two to three times higher than in the general population (48). In another study 4,401 men and women with an age range of 21 to 80 years old, with a mean body mass index (BMI) of 22.6 kg/m2 were enrolled (49). The findings show that men and women with metabolic syndrome are more likely to acquire NAFLD (with an adjusted OR of 4.00 and 11.20, respectively). Furthermore, metabolic syndrome - even in the absence of insulin resistance - increases the risk of CVD about two fold (50).
3.2.5. Lipid Profile and NAFLD
Past studies described serum lipoproteins, lipids, and apolipoprotein levels in individuals diagnosed with NASH (51). They showed that patients with NASH have significantly higher mean serum levels of triglyceride, total cholesterol, and LDL-cholesterol than those of the control group. On the other hand, mean serum HDL-cholesterol concentration was significantly lower than the control group. It seems that liver steatosis does not significantly correlate with decreased mean serum level of Apo AI. However, with a P value of 0.01, it is significantly lower in individuals diagnosed with liver fibrosis in comparison to those without it (51). Studies suggest that the metabolic effects of insulin resistance may contribute to the accumulation of fat in liver tissue, which may promote NAFLD/NASH in patients, resulting in overproduction of lipid particles such as VLDL (52).
Speliotes et al. suggest that dyslipidemia is established independently in patients with fatty liver disease regardless of their visceral fat deposits (53). Another study was conducted on 67 individuals diagnosed with type 2 diabetes, who were withdrawn from anti-diabetic medications before the start of the study (54). The results indicated that fatty liver in individuals with type 2 diabetes had no effect on ApoB levels, but it might potentially promote atherogenesis by raising the mean blood level of triglycerides and small, dense LDL. This, alongside a reduction in mean HDL levels are factors that predispose patients to further CVD.
4. Conclusions
Due to the high prevalence of metabolic disorders and other risk factors, NAFLD has become an extremely important disease in both developed and developing countries. This article addressed the increasing risk of cardiovascular diseases in the context of NAFLD, describing several mediating factors. Since the compact of age and sex varies on the incidence of NAFLD and CVD, further research needs to be conducted to evaluate age and sex as independent risk factors for this disease. Similarly, further meta-analysis can clarify the role of obesity and diabetes on the incidence of CVD in the context of NAFLD, as independent risk factors, not confounding factors. In addition, NAFLD could be an independent cause for CVD. Patients with NAFLD are more likely to have coronary atherosclerosis regardless of the usual causes of CVD, including visceral adiposity. Moreover, with progression to NASH and liver fibrosis, there is an increased risk of CVD, probably through its effect on the hepato-cardiovascular axis. Not surprisingly, the treatment for NAFLD and consequent risk of CVD aim at the same target, mainly by modifying lifestyle in order to decrease insulin tolerance. At last, although it is not possible to answer exactly the question “When should we investigate ischemic heart disease in nonalcoholic fatty liver disease patients?“ because of lack of data, but since cardiovascular events are the main cause of mortality in NAFLD patients, monitoring for CVD and proper pharmacotherapy is essential in individuals with severe NAFLD and those who are at increased conventional risk for ischemic heart disease.
References
-
1.
Lazo M, Clark JM. The epidemiology of nonalcoholic fatty liver disease: a global perspective. Semin Liver Dis. 2008;28(4):339-50. [PubMed ID: 18956290]. https://doi.org/10.1055/s-0028-1091978.
-
2.
Baran B, Akyuz F. Non-alcoholic fatty liver disease: what has changed in the treatment since the beginning? World J Gastroenterol. 2014;20(39):14219-29. [PubMed ID: 25339808]. [PubMed Central ID: PMC4202350]. https://doi.org/10.3748/wjg.v20.i39.14219.
-
3.
Federico A, Dallio M, Masarone M, Persico M, Loguercio C. The epidemiology of non-alcoholic fatty liver disease and its connection with cardiovascular disease: role of endothelial dysfunction. Eur Rev Med Pharmacol Sci. 2016;20(22):4731-41. [PubMed ID: 27906428].
-
4.
Ong JP, Younossi ZM. Epidemiology and natural history of NAFLD and NASH. Clin Liver Dis. 2007;11(1):1-16. [PubMed ID: 17544968]. https://doi.org/10.1016/j.cld.2007.02.009.
-
5.
Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37(4):917-23. [PubMed ID: 12668987]. https://doi.org/10.1053/jhep.2003.50161.
-
6.
Jiang CM, Pu CW, Hou YH, Chen Z, Alanazy M, Hebbard L. Non alcoholic steatohepatitis a precursor for hepatocellular carcinoma development. World J Gastroenterol. 2014;20(44):16464-73. [PubMed ID: 25469014]. [PubMed Central ID: PMC4248189]. https://doi.org/10.3748/wjg.v20.i44.16464.
-
7.
Ekstedt M, Hagstrom H, Nasr P, Fredrikson M, Stal P, Kechagias S, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61(5):1547-54. [PubMed ID: 25125077]. https://doi.org/10.1002/hep.27368.
-
8.
Jahn D, Rau M, Wohlfahrt J, Hermanns HM, Geier A. Non-Alcoholic Steatohepatitis: From Pathophysiology to Novel Therapies. Dig Dis. 2016;34(4):356-63. [PubMed ID: 27170389]. https://doi.org/10.1159/000444547.
-
9.
Shanaki M, Fadaei R, Moradi N, Emamgholipour S, Poustchi H. The Circulating CTRP13 in Type 2 Diabetes and Non-Alcoholic Fatty Liver Patients. PLoS One. 2016;11(12). e0168082. [PubMed ID: 27936230]. [PubMed Central ID: PMC5148106]. https://doi.org/10.1371/journal.pone.0168082.
-
10.
Dyson JK, Anstee QM, McPherson S. Non-alcoholic fatty liver disease: a practical approach to diagnosis and staging. Frontline Gastroenterol. 2014;5(3):211-8. [PubMed ID: 25018867]. [PubMed Central ID: PMC4078666]. https://doi.org/10.1136/flgastro-2013-100403.
-
11.
Kim D, Kim WR, Kim HJ, Therneau TM. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology. 2013;57(4):1357-65. [PubMed ID: 23175136]. [PubMed Central ID: PMC3622816]. https://doi.org/10.1002/hep.26156.
-
12.
Motamed N, Rabiee B, Poustchi H, Dehestani B, Hemasi GR, Khonsari MR, et al. Non-alcoholic fatty liver disease (NAFLD) and 10-year risk of cardiovascular diseases. Clin Res Hepatol Gastroenterol. 2017;41(1):31-8. [PubMed ID: 27597641]. https://doi.org/10.1016/j.clinre.2016.07.005.
-
13.
Zeb I, Li D, Budoff MJ, Katz R, Lloyd-Jones D, Agatston A, et al. Nonalcoholic Fatty Liver Disease and Incident Cardiac Events: The Multi-Ethnic Study of Atherosclerosis. J Am Coll Cardiol. 2016;67(16):1965-6. [PubMed ID: 27102512]. https://doi.org/10.1016/j.jacc.2016.01.070.
-
14.
Francque SM, van der Graaff D, Kwanten WJ. Non-alcoholic fatty liver disease and cardiovascular risk: Pathophysiological mechanisms and implications. J Hepatol. 2016;65(2):425-43. [PubMed ID: 27091791]. https://doi.org/10.1016/j.jhep.2016.04.005.
-
15.
Wu S, Wu F, Ding Y, Hou J, Bi J, Zhang Z. Association of non-alcoholic fatty liver disease with major adverse cardiovascular events: A systematic review and meta-analysis. Sci Rep. 2016;6:33386. [PubMed ID: 27633274]. [PubMed Central ID: PMC5026028]. https://doi.org/10.1038/srep33386.
-
16.
Gordon T, Kannel WB, Hjortland MC, McNamara PM. Menopause and coronary heart disease. The Framingham Study. Ann Intern Med. 1978;89(2):157-61. [PubMed ID: 677576]. https://doi.org/10.7326/0003-4819-89-2-157.
-
17.
Kannel WB. Prevalence and clinical aspects of unrecognized myocardial infarction and sudden unexpected death. Circulation. 1987;75(3 Pt 2):II4-5. [PubMed ID: 3493089].
-
18.
Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367(9524):1747-57. [PubMed ID: 16731270]. https://doi.org/10.1016/S0140-6736(06)68770-9.
-
19.
Fracanzani AL, Tiraboschi S, Pisano G, Consonni D, Baragetti A, Bertelli C, et al. Progression of carotid vascular damage and cardiovascular events in non-alcoholic fatty liver disease patients compared to the general population during 10 years of follow-up. Atherosclerosis. 2016;246:208-13. [PubMed ID: 26803429]. https://doi.org/10.1016/j.atherosclerosis.2016.01.016.
-
20.
Yeung EN, Treskes P, Martin SF, Manning JR, Dunbar DR, Rogers SM, et al. Erratum to: Fibrinogen production is enhanced in an in-vitro model of non-alcoholic fatty liver disease: an isolated risk factor for cardiovascular events? Lipids Health Dis. 2016;15(1):125. [PubMed ID: 27480541]. [PubMed Central ID: PMC4970298]. https://doi.org/10.1186/s12944-016-0290-8.
-
21.
Jin R, Krasinskas A, Le NA, Konomi JV, Holzberg J, Romero R, et al. Association between plasminogen activator inhibitor-1 and severity of liver injury and cardiovascular risk in children with non-alcoholic fatty liver disease. Pediatr Obes. 2018;13(1):23-9. [PubMed ID: 27764892]. https://doi.org/10.1111/ijpo.12183.
-
22.
Pickhardt PJ, Hahn L, Munoz del Rio A, Park SH, Reeder SB, Said A. Natural history of hepatic steatosis: observed outcomes for subsequent liver and cardiovascular complications. AJR Am J Roentgenol. 2014;202(4):752-8. [PubMed ID: 24660702]. https://doi.org/10.2214/AJR.13.11367.
-
23.
Villanova N, Moscatiello S, Ramilli S, Bugianesi E, Magalotti D, Vanni E, et al. Endothelial dysfunction and cardiovascular risk profile in nonalcoholic fatty liver disease. Hepatology. 2005;42(2):473-80. [PubMed ID: 15981216]. https://doi.org/10.1002/hep.20781.
-
24.
Huang PH, Chen JW, Lin SJ. Effects of Cardiovascular Risk Factors on Endothelial Progenitor Cell. Acta Cardiol Sin. 2014;30(5):375-81. [PubMed ID: 27122814]. [PubMed Central ID: PMC4834954].
-
25.
Chiang CH, Huang PH, Chung FP, Chen ZY, Leu HB, Huang CC, et al. Decreased circulating endothelial progenitor cell levels and function in patients with nonalcoholic fatty liver disease. PLoS One. 2012;7(2). e31799. [PubMed ID: 22359630]. [PubMed Central ID: PMC3280999]. https://doi.org/10.1371/journal.pone.0031799.
-
26.
Yilmaz Y, Kurt R, Yonal O, Polat N, Celikel CA, Gurdal A, et al. Coronary flow reserve is impaired in patients with nonalcoholic fatty liver disease: association with liver fibrosis. Atherosclerosis. 2010;211(1):182-6. [PubMed ID: 20181335]. https://doi.org/10.1016/j.atherosclerosis.2010.01.049.
-
27.
Hamaguchi M, Kojima T, Takeda N, Nagata C, Takeda J, Sarui H, et al. Nonalcoholic fatty liver disease is a novel predictor of cardiovascular disease. World J Gastroenterol. 2007;13(10):1579-84. [PubMed ID: 17461452]. [PubMed Central ID: PMC4146902]. https://doi.org/10.3748/wjg.v13.i10.1579.
-
28.
Wong VW, Wong GL, Yip GW, Lo AO, Limquiaco J, Chu WC, et al. Coronary artery disease and cardiovascular outcomes in patients with non-alcoholic fatty liver disease. Gut. 2011;60(12):1721-7. [PubMed ID: 21602530]. https://doi.org/10.1136/gut.2011.242016.
-
29.
Wang CC, Tseng TC, Hsieh TC, Hsu CS, Wang PC, Lin HH, et al. Severity of fatty liver on ultrasound correlates with metabolic and cardiovascular risk. Kaohsiung J Med Sci. 2012;28(3):151-60. [PubMed ID: 22385608]. https://doi.org/10.1016/j.kjms.2011.10.005.
-
30.
Lee JI, Kim MC, Moon BS, Song YS, Han EN, Lee HS, et al. The Relationship between 10-Year Cardiovascular Risk Calculated Using the Pooled Cohort Equation and the Severity of Non-Alcoholic Fatty Liver Disease. Endocrinol Metab (Seoul). 2016;31(1):86-92. [PubMed ID: 26754585]. [PubMed Central ID: PMC4803567]. https://doi.org/10.3803/EnM.2016.31.1.86.
-
31.
Athyros VG, Tziomalos K, Katsiki N, Doumas M, Karagiannis A, Mikhailidis DP. Cardiovascular risk across the histological spectrum and the clinical manifestations of non-alcoholic fatty liver disease: An update. World J Gastroenterol. 2015;21(22):6820-34. [PubMed ID: 26078558]. [PubMed Central ID: PMC4462722]. https://doi.org/10.3748/wjg.v21.i22.6820.
-
32.
Alexander M, Loomis AK, van der Lei J, Duarte-Salles T, Prieto-Alhambra D, Ansell D, et al. Non-alcoholic fatty liver disease and risk of incident acute myocardial infarction and stroke: findings from matched cohort study of 18 million European adults. BMJ. 2019;367:l5367. [PubMed ID: 31594780]. [PubMed Central ID: PMC6780322]. https://doi.org/10.1136/bmj.l5367.
-
33.
Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129(1):113-21. [PubMed ID: 16012941]. https://doi.org/10.1053/j.gastro.2005.04.014.
-
34.
Vishram JK. Prognostic interactions between cardiovascular risk factors. Dan Med J. 2014;61(7):B4892. [PubMed ID: 25123126].
-
35.
Jousilahti P, Vartiainen E, Tuomilehto J, Puska P. Sex, age, cardiovascular risk factors, and coronary heart disease: a prospective follow-up study of 14 786 middle-aged men and women in Finland. Circulation. 1999;99(9):1165-72. [PubMed ID: 10069784]. https://doi.org/10.1161/01.cir.99.9.1165.
-
36.
Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43(2 Suppl 1):S99-S112. [PubMed ID: 16447287]. https://doi.org/10.1002/hep.20973.
-
37.
Targher G, Marra F, Marchesini G. Increased risk of cardiovascular disease in non-alcoholic fatty liver disease: causal effect or epiphenomenon? Diabetologia. 2008;51(11):1947-53. [PubMed ID: 18762907]. https://doi.org/10.1007/s00125-008-1135-4.
-
38.
Targher G, Bertolini L, Poli F, Rodella S, Scala L, Tessari R, et al. Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes. 2005;54(12):3541-6. [PubMed ID: 16306373]. https://doi.org/10.2337/diabetes.54.12.3541.
-
39.
Targher G, Pichiri I, Zoppini G, Trombetta M, Bonora E. Increased prevalence of cardiovascular disease in Type 1 diabetic patients with non-alcoholic fatty liver disease. J Endocrinol Invest. 2012;35(5):535-40. [PubMed ID: 21795844]. https://doi.org/10.3275/7875.
-
40.
Targher G, Bertolini L, Padovani R, Rodella S, Tessari R, Zenari L, et al. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007;30(5):1212-8. [PubMed ID: 17277038]. https://doi.org/10.2337/dc06-2247.
-
41.
Lautamaki R, Borra R, Iozzo P, Komu M, Lehtimaki T, Salmi M, et al. Liver steatosis coexists with myocardial insulin resistance and coronary dysfunction in patients with type 2 diabetes. Am J Physiol Endocrinol Metab. 2006;291(2):E282-90. [PubMed ID: 16478772]. https://doi.org/10.1152/ajpendo.00604.2005.
-
42.
Vasunta RL, Kesaniemi YA, Ylitalo AS, Ukkola OH. High ambulatory blood pressure values associated with non-alcoholic fatty liver in middle-aged adults. J Hypertens. 2012;30(10):2015-9. [PubMed ID: 22940679]. https://doi.org/10.1097/HJH.0b013e3283576faf.
-
43.
Caserta CA, Pendino GM, Amante A, Vacalebre C, Fiorillo MT, Surace P, et al. Cardiovascular risk factors, nonalcoholic fatty liver disease, and carotid artery intima-media thickness in an adolescent population in southern Italy. Am J Epidemiol. 2010;171(11):1195-202. [PubMed ID: 20457571]. https://doi.org/10.1093/aje/kwq073.
-
44.
Lau K, Lorbeer R, Haring R, Schmidt CO, Wallaschofski H, Nauck M, et al. The association between fatty liver disease and blood pressure in a population-based prospective longitudinal study. J Hypertens. 2010;28(9):1829-35. [PubMed ID: 20577126]. https://doi.org/10.1097/HJH.0b013e32833c211b.
-
45.
Lopez-Suarez A, Guerrero JM, Elvira-Gonzalez J, Beltran-Robles M, Canas-Hormigo F, Bascunana-Quirell A. Nonalcoholic fatty liver disease is associated with blood pressure in hypertensive and nonhypertensive individuals from the general population with normal levels of alanine aminotransferase. Eur J Gastroenterol Hepatol. 2011;23(11):1011-7. [PubMed ID: 21915061]. https://doi.org/10.1097/MEG.0b013e32834b8d52.
-
46.
Grundy SM, Brewer HJ, Cleeman JI, Smith SJ, Lenfant C, American Heart A, et al. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109(3):433-8. [PubMed ID: 14744958]. https://doi.org/10.1161/01.CIR.0000111245.75752.C6.
-
47.
Wilson PW, D'Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112(20):3066-72. [PubMed ID: 16275870]. https://doi.org/10.1161/CIRCULATIONAHA.105.539528.
-
48.
Tarantino G, Saldalamacchia G, Conca P, Arena A. Non-alcoholic fatty liver disease: further expression of the metabolic syndrome. J Gastroenterol Hepatol. 2007;22(3):293-303. [PubMed ID: 17295757]. https://doi.org/10.1111/j.1440-1746.2007.04824.x.
-
49.
Hamaguchi M, Kojima T, Takeda N, Nakagawa T, Taniguchi H, Fujii K, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med. 2005;143(10):722-8. [PubMed ID: 16287793]. https://doi.org/10.7326/0003-4819-143-10-200511150-00009.
-
50.
Meigs JB, Rutter MK, Sullivan LM, Fox CS, D'Agostino RS, Wilson PW. Impact of insulin resistance on risk of type 2 diabetes and cardiovascular disease in people with metabolic syndrome. Diabetes Care. 2007;30(5):1219-25. [PubMed ID: 17259468]. https://doi.org/10.2337/dc06-2484.
-
51.
Koruk M, Savas MC, Yilmaz O, Taysi S, Karakok M, Gundogdu C, et al. Serum lipids, lipoproteins and apolipoproteins levels in patients with nonalcoholic steatohepatitis. J Clin Gastroenterol. 2003;37(2):177-82. [PubMed ID: 12869892]. https://doi.org/10.1097/00004836-200308000-00017.
-
52.
Adiels M, Taskinen MR, Packard C, Caslake MJ, Soro-Paavonen A, Westerbacka J, et al. Overproduction of large VLDL particles is driven by increased liver fat content in man. Diabetologia. 2006;49(4):755-65. [PubMed ID: 16463046]. https://doi.org/10.1007/s00125-005-0125-z.
-
53.
Speliotes EK, Massaro JM, Hoffmann U, Vasan RS, Meigs JB, Sahani DV, et al. Fatty liver is associated with dyslipidemia and dysglycemia independent of visceral fat: the Framingham Heart Study. Hepatology. 2010;51(6):1979-87. [PubMed ID: 20336705]. [PubMed Central ID: PMC3023160]. https://doi.org/10.1002/hep.23593.
-
54.
Toledo FG, Sniderman AD, Kelley DE. Influence of hepatic steatosis (fatty liver) on severity and composition of dyslipidemia in type 2 diabetes. Diabetes Care. 2006;29(8):1845-50. [PubMed ID: 16873790]. https://doi.org/10.2337/dc06-0455.
