Abstract
Context:
Bradykinin as a principal active agent of kallikrein-kinin can mediate the powerful cardioprotective mechanism. Both the bradykinin and angiotensin converting enzyme inhibitors (ACE-I) can stimulate this protective pathway through the B2 kinin receptors. We performed a systematic review to determine the effects of bradykinin on cardiac biomarkers and outcomes after surgery or coronary intervention.Evidence Acquisition:
We searched the PubMed, Cochrane CENTRAL, and Scopus databases up to the end of August 2017. Only randomized controlled trials were included.Results:
From a total of 1081 citations, 8 RCTs totaling 268 patients were included for review. Bradykinin could reduce the blood pressure and cause a slight increase in heart rate. But these changes were not significant in comparison to control groups. The major effect of bradykinin or ACE-I on cardiac biomarkers were related to creatine kinase-MB (CKMB). The ST-segment shift was much lower in patients treated with ACE-I as well as the ST-segment peak elevation.Conclusions:
The remote ischemic preconditioning (RIPC) stimulus decreased expression of B1 and B2 kinin receptors and this suggested the role of kinins in cardioprotection. ST-segment shift could be reduced by administration of ACE-I before coronary interventions. In patients who were unresponsive to initial preconditioning ischemia, the infusion of intracoronary enalaprilat during the angioplasty could elicit adequate myocardial protection. The effect of bradykinin is dose dependent. Low doses of bradykinin prevent the increased leukocyte adhesion induced by ischemia reperfusion. High doses activate the bradykinin B2 receptors and lead to increase in leukocyte adhesion. The stimulation of kinin receptor pathway causes a reduction in infarct size, troponin T, peak CKMB levels, and edema of the myocardium. Considering the importance of bradykinin and ACE-I in cardiovascular interventions, further RCTs are required to determine the dosage and precise details of functional pathways of these agents.Keywords
Bradykinin Remote Ischemic Preconditioning Ischemia/Reperfusion Injury Myocardial Protection ACE Inhibitors
1. Context
The heart can be protected against lethal acute ischemia-reperfusion injury by applying cycles of nonlethal ischemia and reperfusion to a remote organ or tissue. This process is called remote ischemic preconditioning (RIPC) (1). Bradykinin as a principal active agent of kallikrein-kinin system, is generated in plasma during endothelial cell injury or within tissues during tissue damage in situations like ischemia-reperfusion injury (2). It has been shown that the stimulation of bradykinin B2 receptors can mediate the powerful cardioprotective mechanism of this molecule. Both the exogenous bradykinin and elevated endogenous bradykinin by inhibition of angiotensin converting enzyme (ACE) share this feature. However, there are some disagreements over the effects of ACE inhibitors and stimulation of B2 receptors in protective effects of remote ischemic preconditioning in man (3, 4). The mechanism of RIPC is not completely understood but some studies suggest that it could be mediated by neural, humoral, or both pathways. It can be beneficial prior to coronary artery bypass graft surgery (CABG), percutaneous coronary revascularization, major vascular surgeries, and also transplantations (5, 6). Based on the importance of RIPC in reducing the ischemic injury to the heart, and the controversial role of bradykinin in this procedure, the aim of this study is performing a systematic review to investigate the effects of bradykinin on cardiac biomarkers and outcomes after surgery or coronary intervention.
2. Evidence Acquisition
We performed a systematic search of published studies in English in the PubMed, Cochrane CENTRAL, and Scopus. The search was limited until the end of August 2017. Key words included ‘remote ischemic preconditioning’, ‘ischemia AND reperfusion’, ‘bradykinin’, ‘ACE inhibitors’, ‘ischemic preconditioning mediators’, ‘cardioprotection AND cardiac surgery’, and ‘percutaneous coronary intervention’. There was no limitation for age and gender of patients to include. We used Endnote software version 8.1 to include non-duplicated citations. Only randomized controlled trials (RCTs) in patients undergoing percutaneous coronary intervention (PCI) or cardiac surgery performed on cardiopulmonary bypass were selected for review. Description studies, review studies, case reports, and RCTs on animals were excluded.
3. Results
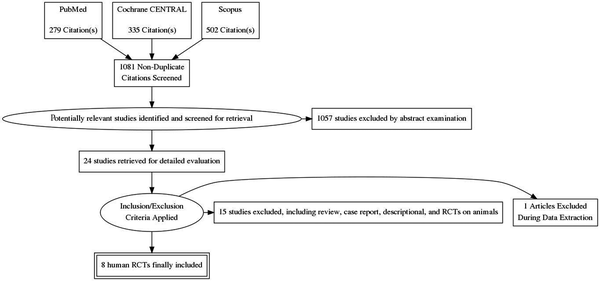
From a total of 1081 citations, 8 RCTs totaling 268 patients were included for review (Figure 1). A summary of interventions and outcomes of these studies is presented in Table 1. Further characteristics of RCTs (7-14) are listed in Table 2. In bradykinin groups, the blood pressure (BP) was smaller than control groups (7, 10, 11) but in one study it had no effect (8). In one study it was stated that the heart rate (HR) was greater in BK group rather than control group. However, the changes in HR was similar in both group (11). There was no significant difference in HR and BP between both groups and changes to these values are dose dependent. The level of ischemic biomarkers of the heart including CKMB, troponin I and T, and LDH were significantly lower in comparison to control groups due to administration of bradykinin or ACE inhibitors. These measures were more prominent for CKMB that suggest less liberation of this enzyme. These values were less important for troponin and LDH (7, 9, 10, 13, 14). Another important subject was the effects of bradykinin on ST-segment alterations. Patients treated with BK or ACE-I during PCI showed less variations in ST-segment shifting. This effect was more visible during the first balloon inflation. The peak of ST-segment was also less than control group (7, 8, 11, 12).
Flow diagram of the selection process of eligible articles
Summary of Data and Conclusions From Included Studies
| Study | Interventions | Results | Conclusion, Recommendations |
|---|---|---|---|
| Boldt et al. (7) | Adm. of enalaprilat after anesthesia in CABG candidates, continuous intravenous infusion of enalaprilat (0.6 micg/kg/min) | Markers of ischemic myocardial tissue damage (CKMB, TnT, and GPBB) increased significantly less than control group. Changes in the ST segment indicating ischemia were least common in the enalaprilat group. | Continuous infusion of ACE inhibitors before cardio-pulmonary bypass may help to protect the heart against ischemia/reperfusion injury. |
| Leesar et al. (8) | Receive a 10-min intracoronary infusion of bradykinin (2.5 mg/min) or normal saline just 10 min before PTCA(three times of 2 min balloon inflations 5 min apart) | In bradykinin-treated patients, the ST-segment shift during the first inflation was significantly smaller than in the control group, and there were no appreciable differences in ST-segment shift during the three inflations. | Infusion of bradykinin had no hemodynamic effects and no significant adverse effects, and it could protect the myocardium against the ischemia. |
| Walter et al. (9) | Adm. of 7.5 mg/d enalapril on first day and 20 mg/d on the other 6 days (mean) before the CABG | No significant differences between enalapril and control group concerning CK, CK-MB, LDH, TnT, TAT, fibrinogen, and kallikrein like activity. | ACE inhibition before CABG is feasible without activation of contact phase. It has no effect on the time course of thrombin activation. Reduction of ischemic injury during CPB is not achieved by enalapril. |
| Wei et al. (10) | Total dose of 25μg of BK infusion for 7 min prior to cardiopulmonary bypass | Acute decrease of blood pressure, minimum MAP was 72.7% of the original MAP. No difference in baseline levels of cTnI and CK-MB between case and control group. BK group released less CK-MB.(lower maximum CK-MB). | Exogenous bradykinin is weak cardioprotective agent in low-risk patients. This dose cause acute reduction in BP. |
| Leesar et al. (11) | Intracoronary enalaprilat or normal saline before PTCA. Enalaprilat (0.75 mg in 50 mL saline) was infused over 15 min at 0.05 mg/min directly into the stenotic artery (total dose 0.75 mg) | Greater ST-segment shift in control group during the first inflation than during the second and third inflations. Enalaprilat-pretreated patients showed no change in ST-segment shift during inflations. Smaller chest pain score in enalaprilat group. | Pretreatment with enalaprilat attenuates the manifestations of myocardial ischemia during angioplasty. |
| Ungi et al. (12) | Two 120-second coronary artery occlusions separated by a reperfusion interval of 10 min. Intracoronary infusion of enalaprilat (50 μg/min) between inflations | No significant differences in ST-segment elevation between two inflations in control group. Less pronounced and slower ST-segment elevation before second inflation than the first one in enalaprilat group. | Adm. of enalaprilat during PCI provides protection to patients who do not have a protective response to the initial balloon inflation. |
| Wang et al. (13) | Infusion of 25 μg BK via central venous port of a Swan-Ganz catheter for 7 min before the CPB | No significant difference in TnI between groups. Patients released significantly less CK-MB than the controls. plasma levels of IL-6, IL-8 and IL-10 increased significantly after reperfusion in both groups as compared with baseline. | Exogenous adm. of BK prior to CPB in CABG patients attenuates ischemic myocardial injury. shifting the circulating inflammatory cytokine balance towards the anti-inflammatory direction. |
| Saxena et al. (14) | RIPC by inflation of a blood pressure cuff on the arm | Kinin B2 receptor expression did not differ between the groups at baseline (pre-RIPC), but was significantly lower in the RIPC group than in the control group after RIPC/sham. Down-regulation of both B1 and B2 receptors in RIPC group. No differences in CK, CRP, cytokine, lactate or troponin I levels between the groups. Increase in neutrophil elastase levels. | RIPC down-regulated the expression of kinin B1 and B2 receptors in neutrophils of patients undergoing CABG. |
Data and Characteristics of the Patients
| Study | Year | Design | No. | M | Age | BP (MAP) | HR | CKMB (U/L) | TnT (μg/L) | LDH (U/L) | ST-Elevation | Anesthesia |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boldt et al. (7) | 1996 | RCT | (TnT) | Midazolam, fentanyl, pancuronium | ||||||||
| Case | 22 | NA | 63.2 ± 6.6 | Reduce | No effect | 7.5 (2.3) | 0.71 | NA | No | |||
| Control | 22 | NA | 62.9 ± 6.0 | No effect | No effect | 34.2 (5.8) (#2MI) | 1.01 | NA | #15 | |||
| Leesar et al. (8) | 1999 | RCT | ST-shift | Midazolam | ||||||||
| Case | 15 | 11 | 59 ± 3 | No effect | No effect | NA | NA | NA | Smaller | |||
| Control | 15 | 12 | 60 ± 3 | No effect | No effect | NA | NA | NA | Greater | |||
| Walter et al. (9) | 2002 | RCT | NA | |||||||||
| Case | 22 | 17 | 64.8 ± 1.7 | NA | NA | 318 ± 38.6 | 1.6 | 478.4 ± 47.5 | NA | |||
| Control | 21 | 16 | 61.9 ± 2. | NA | NA | 316 ± 16.8 | 1.4 | 515.3 ± 99.3 | NA | |||
| Wei et al. (10) | 2004 | RCT | cTnI | Propofol, sufentanil, rocuronium | ||||||||
| Case | 21 | 19 | 65.6 ± 7 | 74.7 ± 7.9 | No effect | 19.5 ± 11.5 | 6.1 ± 4.7 | NA | NA | |||
| Control | 20 | 18 | 68.8 ± 8.7 | - | No effect | 28.7 ± 23.8 | 7.3 ± 9.0 | NA | NA | |||
| Leesar et al. (11) | 2007 | RCT | ST-shift | NA | ||||||||
| Case | 11 | 9 | 54 ± 3 | 127 ± 6 | 73±4 | NA | NA | NA | No | |||
| Control | 11 | 7 | 50 ± 3 | 131 ± 8 | 71±3 | NA | NA | NA | Greater | |||
| Ungi et al. (12) | 2008 | RCT | NA | |||||||||
| Case | 10 | 5 | 58 ± 7 | NA | NA | NA | NA | NA | Less ST peak | |||
| Control | 10 | 6 | 52 ± 6 | NA | NA | NA | NA | NA | Higher ST peak | |||
| Wang et al. (13) | 2009 | RCT | (TnI) | Propofol, sufentanil, rocuronium, midazolam | ||||||||
| Case | 19 | 17 | 66.0 ± 1.6 | NA | NA | 5.7 ± 0.3 | 0.21 ± 0.01 | NA | NA | |||
| Control | 19 | 17 | 68.4 ± 2.1 | NA | NA | 5.8 ± 0.4 | 0.24 ± 0.03 | NA | NA | |||
| Saxena et al. (14) | 2013 | RCT | (TnI) | No differences | NA | |||||||
| Case | 15 | 15 | 65.1 ± 10.5 | NA | NA | 381.5 | 2.28 | NA | ||||
| Control | 15 | 13 | 68.7 ± 7.8 | NA | NA | 408.1 | 2.94 | NA |
4. Conclusions
The role of bradykinin in cardioprotection was first described by Wall et al. in 1994. By means of HOE140 (a bradykinin B2 receptor antagonist) they showed that the endogenous bradykinin may mediate the cardioprotective events associated with ischemic preconditioning (15). Brew et al. explained that ischemic preconditioning by transient ischemia involved in intrinsic cardiac bradykinin receptor stimulation and stimulation of B2 receptors, trigger a series of events which lead to the activation of protein kinase C (16). Alterations in tissue blood flow and a reduction in plasma pH can increase the bradykinin concentration by activation of plasma kallikrein and reduce kinin breakdown. Schulz et al. demonstrated that preconditioning ischemia/reperfusion was related to reduction in infarct size and bradykinin was essential during preconditioning ischemia of shorter duration (17). Pan et al. showed that treatment with captopril resulted in an increased myocardial interstitial bradykinin accumulation in the ischemic zone above and beyond the bradykinin level produced by ischemia alone. They demonstrated that the bradykinin level was greater in the endocardium than in the epicardium during ischemia (18). Captopril can potentiate ischemic preconditioning through B2 receptor activation without increasing the arterial kinin level (19). In 2000, Sato et al. demonstrated that losartan as an angiotensin II type 1-receptor blocker was resulted in a reduction in myocardial infarct size and apoptotic cell death. Losartan provided cardioprotection through both bradykinin-dependent and bradykinin-independent mechanisms and it was completely blocked by HOE 140. This study supported the role of bradykinin B2 receptor in ischemic preconditioning (20). Schriefer et al. explained that the combination of ramiprilat and cFP-AAF-pAB, an endopeptidase inhibitor, significantly increased tissue bradykinin level. Inhibition of bradykinin -inactivating enzymes protects endogenous bradykinin from degradation and provides long-lasting protection from myocardial ischemia/reperfusion injury (21). These were examples of experiment on animals.
In the setting of human investigations, we will describe the randomized controlled trials here. Boldt et al. demonstrated that enalaprilat relieved myocardial ischemia after MI and could protect the myocardium before ischemia. ST-segment changes as an indicator of ischemia was least common in the group that treated with enalaprilat. Enalaprilat-treated patients showed the smallest overall changes in standard CKMB and TnT (7). Leesar et al. in 1999, demonstrated the effect of 10-min intracoronary infusion of bradykinin before percutaneous transluminal coronary angioplasty. It was showed that the ST-segment shift during the first inflation was significantly smaller than in the control group, and there were no appreciable differences in ST-segment shift during the three inflations. In this study it was stated that bradykinin had no hemodynamic effects (BP and HR changes) and no significant adverse effects (8). Walter et al. examined the effect of oral ACE inhibitors on kallikrein contact phase and hemostasis during cardiopulmonary bypass. There were no significant difference between enalaprilat and placebo group concerning CKMB, TnT, and LDH. Fibrinogen levels significantly elevated during the post-operative follow up in both groups (9). Study of Wei et al. demonstrated that infusion of 25μg bradykinin before initiation of cardiopulmonary bypass (CPB) could have led to acute decrease of blood pressure with a slight increase in heart rate. Cardiac index (CI) also increased 30-min after CPB. Among the cardiac biomarkers, only CKMB was significantly less in the controls postoperatively. It indicated less myocardial injury after coronary artery bypass grafting surgery (10). Leesar et al. in 2007, demonstrated the first evidence showing that ACE inhibitors are cardioprotective in human during angioplasty. They used direct infusion of enalaprilat into stenotic artery, followed by preconditioning protocol. Enalaprilat-pretreated patients showed no change in ST-segment shift during inflations on either the intracoronary or the surface ECG. ST-segment shift and chest pain score was also lower in comparison to placebo group (11). Ungi et al. demonstrated that the infusion of enalaprilat significantly decreased the mean ST elevation from the first to the second occlusion during PCI. The peak ST elevation was also reduced during the second inflation in enalaprilat group. They showed that in patients who were unresponsive to initial preconditioning ischemia, the infusion of intracoronary enalaprilat during the angioplasty could elicit adequate myocardial protection (12). Wang et al. explained that bradykinin could cause less CKMB to be released. From the perspective of anti-inflammatory role of bradykinin, the ratio of IL-8 to IL-10 was significantly lower in BK groups than in controls. IL-10 inhibits the production of pro-inflammatory cytokines and this strategy may attenuate the postoperative myocardial injury and improve the heart function (13). In recent study, Saxena et al. demonstrated the effect of remote ischemic preconditioning (RIPC) on kinin receptor expression. Kinin B2 receptor expression was significantly lower in the RIPC group than in the control group. Expressions of both kinin B1 and B2 receptors were significantly down-regulated in the RIPC group, and this persisted to 24 h after surgery. RIPC had no effect on post-operation levels of neutrophil elastase. There were no differences between the RIPC and control groups in the levels of IL-6, IL-8, IL-10 or TNF-α and also in CK, CRP, cytokine, lactate or troponin I levels (14).
Cardiac surgery and percutaneous coronary interventions have the potential for ischemia and reperfusion injury to the heart and other vital organs. RIPC before cardiac surgery results in reductions in biomarkers of renal and cardiac injury (22). Following acute myocardial infarction, reactive oxygen species (ROS) is enhanced in myocardium and oxidative stress is developed in both infarcted and non-infarcted myocardium. Activation of bradykinin B2 receptors during ischemic post-conditioning may lead to protection via reactive oxygen species (ROS) signaling. This signal is abolished by ROS scavenger like N-acetyl-L-cysteine intermittent bradykinin accumulation and ROS compartmentalization are playing a role in myocardial protection during reperfusion (23, 24). The effects of bradykinin on leukocyte rolling and adhesion are highly concentration dependent. High doses activate the bradykinin B2 receptors and leukocyte adhesion increased. But low doses of bradykinin prevent the increased leukocyte adhesion induced by ischemia reperfusion by a mechanism that involves B2-receptor activation and the formation of nitric oxide (25). The RIPC stimulus decreased expression of B1 and B2 kinin receptors on circulating human neutrophils for at least 24 h. This subject suggest the role of kinin receptors in RIPC. However, the role of this inflammatory pathway in RIPC and organ protection need further investigations (14, 26). The study of Liuba et al. suggested that bradykinin can protect the arterial endothelial function against ischemia/reperfusion injury by preserving the endothelial NO availability (27). RIPC save the myocardium from infarction in ST-segment elevation MI (STEMI) patients treated with primary PCI. It causes a reduction in infarct size, troponin T, peak CKMB levels, and edema of the myocardium. The clinical effect of RIPC is independent on infarct location and is considered as low-risk treatment for all patients with STEMI (28-30).
4.1. Conclusions
Remote ischemic preconditioning as a cardioprotective strategy can reduce the infarct size in patients with STEMI during ischemic/reperfusion injury. One of its mediators is bradykinin that act through B1 and B2 receptors. These receptors play a key role in ischemic inflammatory pathway and they are stimulated due to administration of ACE inhibitors or exogenous bradykinin. This pathway leads to reduction in cardiac biomarkers like CKMB and less shift in ST-segment which reflects the electrocardiogram pattern of ischemia. It was showed that the administration of bradykinin and ACE inhibitors are beneficial for patients that are candidate for cardiovascular surgery or PCI, but further RCTs are required to determine the dosage and Precise details of functional pathways of these agents.
References
-
1.
Hausenloy DJ, Candilio L, Evans R, Ariti C, Jenkins DP, Kolvekar S, et al. Remote ischemic preconditioning and outcomes of cardiac surgery. N Engl J Med. 2015;373(15):1408-17. [PubMed ID: 26436207]. https://doi.org/10.1056/NEJMoa1413534.
-
2.
Sharma R, Randhawa PK, Singh N, Jaggi AS. Bradykinin in ischemic conditioning-induced tissue protection: Evidences and possible mechanisms. Eur J Pharmacol. 2015;768:58-70. [PubMed ID: 26499976]. https://doi.org/10.1016/j.ejphar.2015.10.029.
-
3.
Jancso G, Jaberansari M, Gasz B, Szanto Z, Cserepes B, Roth E. Bradykinin and angiotensin-converting enzyme inhibition in cardioprotection. Exp Clin Cardiol. 2004;9(1):21-5. [PubMed ID: 19641692]. [PubMed Central ID: PMC2716258].
-
4.
Pedersen CM, Schmidt MR, Barnes G, Botker HE, Kharbanda RK, Newby DE, et al. Bradykinin does not mediate remote ischaemic preconditioning or ischaemia-reperfusion injury in vivo in man. Heart. 2011;97(22):1857-61. [PubMed ID: 21873443]. https://doi.org/10.1136/heartjnl-2011-300323.
-
5.
Walsh SR, Tang T, Sadat U, Dutka DP, Gaunt ME. Cardioprotection by remote ischaemic preconditioning. Br J Anaesth. 2007;99(5):611-6. [PubMed ID: 17905751]. https://doi.org/10.1093/bja/aem273.
-
6.
Morris P, Knechtle S. Immunology of Graft Rejection. Kidney transplantation: principles and practice. 7th ed. Philadelphia: Elsevier; 2014.
-
7.
Boldt J, Rothe G, Schindler E, Doll C, Gorlach G, Hempelmann G. Can clonidine, enoximone, and enalaprilat help to protect the myocardium against ischaemia in cardiac surgery? Heart. 1996;76(3):207-13. [PubMed ID: 8868976]. [PubMed Central ID: PMC484507].
-
8.
Leesar MA, Stoddard MF, Manchikalapudi S, Bolli R. Bradykinin-induced preconditioning in patients undergoing coronary angioplasty. J Am Coll Cardiol. 1999;34(3):639-50. [PubMed ID: 10483942].
-
9.
Walter T, Helber U, Bail D, Heller W, Hoffmeister HM. Influence of ACE inhibition on myocardial damage, the Kallikrein-Kinin system and hemostasis during cardiopulmonary bypass surgery. Thorac Cardiovasc Surg. 2002;50(3):150-4. [PubMed ID: 12077687]. https://doi.org/10.1055/s-2002-32409.
-
10.
Wei M, Wang X, Kuukasjarvi P, Laurikka J, Rinne T, Honkonen EL, et al. Bradykinin preconditioning in coronary artery bypass grafting. Ann Thorac Surg. 2004;78(2):492-7. [PubMed ID: 15276504]. https://doi.org/10.1016/j.athoracsur.2003.11.039.
-
11.
Leesar MA, Jneid H, Tang XL, Bolli R. Pretreatment with intracoronary enalaprilat protects human myocardium during percutaneous coronary angioplasty. J Am Coll Cardiol. 2007;49(15):1607-10. [PubMed ID: 17433950]. https://doi.org/10.1016/j.jacc.2007.01.060.
-
12.
Ungi I, Palinkas A, Nemes A, Ungi T, Thury A, Sepp R, et al. Myocardial protection with enalaprilat in patients unresponsive to ischemic preconditioning during percutaneous coronary intervention. Can J Physiol Pharmacol. 2008;86(12):827-34. [PubMed ID: 19088803]. https://doi.org/10.1139/Y08-096.
-
13.
Wang X, Wei M, Kuukasjarvi P, Laurikka J, Rinne T, Moilanen E, et al. The anti-inflammatory effect of bradykinin preconditioning in coronary artery bypass grafting (bradykinin and preconditioning). Scand Cardiovasc J. 2009;43(1):72-9. [PubMed ID: 18609065]. https://doi.org/10.1080/14017430802180449.
-
14.
Saxena P, Aggarwal S, Misso NL, Passage J, Newman MA, Thompson PJ, et al. Remote ischaemic preconditioning down-regulates kinin receptor expression in neutrophils of patients undergoing heart surgery. Interact Cardiovasc Thorac Surg. 2013;17(4):653-8. [PubMed ID: 23814135]. [PubMed Central ID: PMC3781800]. https://doi.org/10.1093/icvts/ivt279.
-
15.
Wall TM, Sheehy R, Hartman JC. Role of bradykinin in myocardial preconditioning. J Pharmacol Exp Ther. 1994;270(2):681-9. [PubMed ID: 8071859].
-
16.
Brew EC, Mitchell MB, Rehring TF, Gamboni-Robertson F, McIntyre RC Jr, Harken AH, et al. Role of bradykinin in cardiac functional protection after global ischemia-reperfusion in rat heart. Am J Physiol. 1995;269(4 Pt 2):H1370-8. [PubMed ID: 7485570]. https://doi.org/10.1152/ajpheart.1995.269.4.H1370.
-
17.
Schulz R, Post H, Vahlhaus C, Heusch G. Ischemic preconditioning in pigs: a graded phenomenon: Its relation to adenosine and bradykinin. Circulation. 1998;98(10):1022-9. [PubMed ID: 9737523].
-
18.
Pan HL, Chen SR, Scicli GM, Carretero OA. Cardiac interstitial bradykinin release during ischemia is enhanced by ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2000;279(1):H116-21. [PubMed ID: 10899048]. https://doi.org/10.1152/ajpheart.2000.279.1.H116.
-
19.
Miki T, Miura T, Ura N, Ogawa T, Suzuki K, Shimamoto K, et al. Captopril potentiates the myocardial infarct size-limiting effect of ischemic preconditioning through bradykinin B2 receptor activation. J Am Coll Cardiol. 1996;28(6):1616-22. [PubMed ID: 8917279].
-
20.
Sato M, Engelman RM, Otani H, Maulik N, Rousou JA, Flack JE 3rd, et al. Myocardial protection by preconditioning of heart with losartan, an angiotensin II type 1-receptor blocker: Implication of bradykinin-dependent and bradykinin-independent mechanisms. Circulation. 2000;102(19 Suppl 3):III346-51. [PubMed ID: 11082412].
-
21.
Schriefer JA, Broudy EP, Hassen AH. Inhibitors of bradykinin-inactivating enzymes decrease myocardial ischemia/reperfusion injury following 3 and 7 days of reperfusion. J Pharmacol Exp Ther. 2001;298(3):970-5. [PubMed ID: 11504792].
-
22.
Pierce B, Bole I, Patel V, Brown DL. Clinical outcomes of remote ischemic preconditioning prior to cardiac surgery: A meta-analysis of randomized controlled trials. J Am Heart Assoc. 2017;6(2). [PubMed ID: 28219918]. [PubMed Central ID: PMC5523764]. https://doi.org/10.1161/JAHA.116.004666.
-
23.
Sun Y. Myocardial repair/remodelling following infarction: Roles of local factors. Cardiovasc Res. 2009;81(3):482-90. [PubMed ID: 19050008]. [PubMed Central ID: PMC2639132]. https://doi.org/10.1093/cvr/cvn333.
-
24.
Penna C, Mancardi D, Rastaldo R, Losano G, Pagliaro P. Intermittent activation of bradykinin B2 receptors and mitochondrial KATP channels trigger cardiac postconditioning through redox signaling. Cardiovasc Res. 2007;75(1):168-77. [PubMed ID: 17400201]. https://doi.org/10.1016/j.cardiores.2007.03.001.
-
25.
Shigematsu S, Ishida S, Gute DC, Korthuis RJ. Concentration-dependent effects of bradykinin on leukocyte recruitment and venular hemodynamics in rat mesentery. Am J Physiol. 1999;277(1 Pt 2):H152-60. [PubMed ID: 10409193].
-
26.
Saxena P, Shaw OM, Misso NL, Naran A, Shehatha J, Newman MA, et al. Remote ischemic preconditioning stimulus decreases the expression of kinin receptors in human neutrophils. J Surg Res. 2011;171(1):311-6. [PubMed ID: 20189583]. https://doi.org/10.1016/j.jss.2009.11.011.
-
27.
Liuba P, Batra S, Pesonen E, Werner O. Bradykinin preconditions postischemic arterial endothelial function in humans. J Card Surg. 2005;20(5):420-4. [PubMed ID: 16153271]. https://doi.org/10.1111/j.1540-8191.2005.2004120.x.
-
28.
White SK, Frohlich GM, Sado DM, Maestrini V, Fontana M, Treibel TA, et al. Remote ischemic conditioning reduces myocardial infarct size and edema in patients with ST-segment elevation myocardial infarction. JACC Cardiovasc Interv. 2015;8(1 Pt B):178-88. [PubMed ID: 25240548]. https://doi.org/10.1016/j.jcin.2014.05.015.
-
29.
Prunier F, Angoulvant D, Saint Etienne C, Vermes E, Gilard M, Piot C, et al. The RIPOST-MI study, assessing remote ischemic perconditioning alone or in combination with local ischemic postconditioning in ST-segment elevation myocardial infarction. Basic Res Cardiol. 2014;109(2):400. [PubMed ID: 24407359]. https://doi.org/10.1007/s00395-013-0400-y.
-
30.
Sloth AD, Schmidt MR, Munk K, Kharbanda RK, Redington AN, Schmidt M, et al. Improved long-term clinical outcomes in patients with ST-elevation myocardial infarction undergoing remote ischaemic conditioning as an adjunct to primary percutaneous coronary intervention. Eur Heart J. 2014;35(3):168-75. [PubMed ID: 24031025]. https://doi.org/10.1093/eurheartj/eht369.