Abstract
Background:
Bipolar I disorder (BP-I) is one of the significant disabling psychiatric disorders resulting in severe deficits in the social and personal function of suffering patients. Among its etiologies, immunologic and genetic disturbances are two important areas of interest.Objectives:
This study aimed to assess the potential role of interleukin-1β (IL-1β)-511 polymorphism in BP-I pathogenesis based on a previous pilot study.Methods:
After diagnostic interviews held by two psychiatrists using structured clinical interview for DSM disorder (SCID), 102 bipolar-diagnosed hospitalized patients in Ibn-e-Sina Hospital, Mashhad, Iran, were selected and compared with 102 healthy individuals of the control group. The DNA was extracted from the blood samples of each group. Genetic locus -511 of IL-1β was defined by its specific primers. Polymerase chain reaction (PCR) and restriction fragment length polymorphism (RFLP) were also carried out. The two groups’ results were compared by SPSS-20 using the chi-square test.Results:
There were no significant differences in the genotypic frequency of IL-1β locus -511 (P = 1) and C/T allelic frequency (P = 0.42) between bipolar and control groups. There was also no significant difference in the allelic frequency between psychotic and non-psychotic subgroups (P = 0.218) and suicidal and non-suicidal subgroups of bipolar patients (P = 0.829). The genotypic distribution of -511 IL-1β polymorphisms in the control group was in the Hardy-Weinberg equilibrium.Conclusions:
In contrast with a previous pilot study, this study found no relationship between BP-I and genotypic and C/T allelic frequencies of -511 IL-1β polymorphism. There were also no associations between the allelic frequency and two subgroups of psychotic/non-psychotic and suicidal/non-suicidal of bipolar patients.Keywords
1. Background
Bipolar disorder (BP) is one of the major psychiatric disorders that can severely affect patients’ personal and social life and cause loss of disability-adjusted life years (DALY) even more than what happens in all different kinds of cancer or some important neurologic diseases such as Alzheimer’s disease and epilepsy (1, 2). Its prevalence is estimated at approximately 1.0% in the general population (1, 3), with sibling risk recurrence around 7 - 10 and heritability near 80% - 90% (4). Further, the pattern of its heritability seems to be complex referring to the non-mendelian types of transmission with incomplete genetic penetrance and polygenetic inheritability (5). Pathogenesis is still unknown, but in recent years there has been too much focus on neurodegenerative and neurodevelopmental phenomena regarding the potential role of inflammation in the regulation of neuronal structure and neuronal function (6).
A growing body of evidence supports the role of the inflammatory system in bipolar pathogenesis and indicates that the increased level of pro-inflammatory markers is associated with a bipolar mood disorder (7-9) such as interleukin 1 and 6 (10). These polypeptide agents play different roles in the immune system secreted by a variety of cells such as endothelial cells, T lymphocytes, and macrophages which influences vascular permeability, called neutrophils to the inflammation sites, and provokes the synthesis of different agents such as free radicals in those specific regions. Along with its protective role against different causations, it could also lead to anatomical injuries and cell death even in the CNS. The association between different episodes of bipolar disorder and pro-inflammatory situations was indicated in different studies (11).
Among inflammatory cytokines, interleukin-1 beta (IL-1β) is reported to be involved in mesencephalic progenitor cells’ differentiation into dopaminergic neuronal cells (12, 13). It also plays a role in acute and chronic neurodegenerative outcomes and neurodevelopmental processes in the embryonic period by provoking the synthesis of some factors such as nerve growth factor (NGF) and inhibiting the expression of brain-derived neurotrophic factor (BDNF) (13), which is one of the suspicious sites for scientists who investigate bipolar psychopathogenesis.
Compatible with these considerations, a systemic review stated that inflammation may be related to bipolar disorder in some different areas (14). Another study indicated that the mechanisms involved in the immune system may be associated with BP core psychopathology resulting from the relations between affective symptoms of BP and markers of inflammation (15). Another study manifested that in the acute phase of mania, we may face active inflammation and elevated levels of IL-1RA, sTNF-R1, and hs-CRP. Also, chronic inflammation could be seen in both the acute phase by the increased levels of hs-CRP and IL-1RA and the full remission period of mania by the sustained higher levels of hs-CRP (16). Furthermore, in the depressive episode of BP, persistent inflammation and increased levels of IL-1RA, hs-CRP, sIL-2R, and sTNF-R1 could be seen initiating in the acute phase and continuing to full remission (17).
According to molecular genetic studies, there could be an association between some immunologic-related genes and bipolar disorder (10). Existing evidence suggests an association between inflammation and bipolar disorder by common genetic polymorphisms (14). A study supported the role of genes involved in cytokines encoding associated with dysregulations in the immune system, presented by pathological decreasing or increasing of cytokine components in mood disorders (9). Another study suggests that this dysregulation in inflammatory cascade, presented by decreasing or increasing inflammatory products, may be associated with genetic-based variations in BP patients (6). In another study, an association was found between BP and the variable number of tandem repeats (VNTR) polymorphism in the interleukin-1 receptor antagonist (IL-1RA) gene, which serves as an important regulator of the activity of interleukin-1β (IL-1β) and interleukin-1 α (IL-1α) (18).
In contrast, a study revealed no significant differences in genotypic or allelic frequencies between bipolar patients and the control group (13). Another study reported no significant differences in allelic frequency and genotypic distribution of IL-1RA gene polymorphism between bipolar patients and the control group (19). Additionally, another study on the -308 promoter polymorphism of tumor necrosis factor-alpha (TNF-α) as a pro-inflammatory cytokine did not support the impact of this given polymorphism on bipolar pathogenesis (20).
According to the potential role of cytokine-related genes in the pathogenesis of bipolar disorder and significant probability of IL-1 involvement, a pilot study was carried out in Mashhad Ibn-e-Sina Hospital, Iran, comparing the polymorphism of the IL-1 cluster in four loci (-511 IL-1β, +3954 IL-1β, -889 IL-1α, and IL-1RN) between bipolar and control groups. As the results showed, significant differences were seen in the CC genotypic frequency (P = 0.04) and C/T allelic frequency (P = 0.02) just in one locus (-511 IL-1β) between 48 bipolar patients and 47 healthy individuals (21).
2. Objectives
This study aimed to compare the polymorphism of IL-1β in this specific locus (-511 IL-1β) between bipolar and control groups, evaluating the potential association between -511 IL-1β polymorphism and BP-I by selecting a larger sample size based on statistic assessments.
3. Methods
Based on the information obtained from the preliminary research conducted by Talaei et al. (21), the sample size was calculated to be 98 individuals in each group, with a 0.95 confidence level, 80% test power, and 0.16 accuracy. Considering the possibility of participants’ withdrawal from the study, 105 individuals in each group entered the study. With the withdrawal of three participants in each group due to dissatisfaction with continuing the study, finally, 102 individuals completed the study in each of the bipolar and control groups.
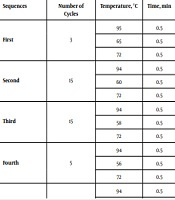
In this study, after diagnostic interviews held by two psychiatrists using structured clinical interview for DSM disorder (SCID), finally, 102 bipolar-diagnosed hospitalized Iranian volunteers in Mashhad Ibn-e-Sina Hospital, Iran, were included as the case group after ruling out immunologic, genetic, thyroid, and neurologic diseases and substance-induced mood disorders. They were compared with 102 healthy unrelated volunteer staff of Ibn-e-Sina Hospital and Bu Ali Research Institute, without personal and familial history of mood disorders included through the same inclusion criteria. The exclusion criterion was the dissatisfaction of participants to continue the study. The participants of each group were selected by a convenience method of sampling. After obtaining informed consent, a 10 cc blood sample was collected from each individual. The DNA samples were extracted at the Bu-Ali research center, and IL-1β locus -511 (placed in the promotor region) was defined by its specific primers with the sequences of forward 5’-GCC TGA ACC CTG GAT ACC GT-3’ and reverse 5’-GCC AAT AGC CCT CCC TGT CT- 3’. Next, the distribution of biallelic polymorphism (C → T replacement) was assessed. Then, polymerase chain reaction (PCR) was carried out, respectively, as seen in Table 1. The resulting products underwent electrophoresis on a 3% agarose gel, stained by ethidium bromide for making visible the 155bp bands. Restriction fragment length polymorphism (RFLP) was carried out by using restriction enzyme endonuclease Ava I (Ferments, Karaj) and incubated at 37°C overnight. This enzyme cut the length of DNA at its restriction site, 5’…C↓PyCGPuG…3’. After finishing the incubation period, to deactivate the enzyme, 1 µL of 0.05% EDTA was used at 85°C temperature for one hour. If the restriction site was present, the main segment with the length of 155 bp was divided into two 88 bp and 67 bp segments, which implicated on the C allele (wild allele or allele 1). If the restriction site was not present, the 155 bp segment remained intact and undivided, which implicated on the T allele (variant allele or allele 2). Thus, the CC, CT, and TT genotypes were defined. For better vision, RLEP products were electrophoresis on the 17% polyacrylamide gel and stained with silver nitrate. Then, the results of each group were compared statistically by SPSS version 20 software using chi-square (χ2). The P-value < 0.05 was considered statistically significant.
Sequences of PCR in Case and Control Groups
| Sequences | Number of Cycles | Temperature, °C | Time, min |
|---|---|---|---|
| First | 3 | 95 | 0.5 |
| 65 | 0.5 | ||
| 72 | 0.5 | ||
| Second | 15 | 94 | 0.5 |
| 60 | 0.5 | ||
| 72 | 0.5 | ||
| Third | 15 | 94 | 0.5 |
| 58 | 0.5 | ||
| 72 | 0.5 | ||
| Fourth | 5 | 94 | 0.5 |
| 56 | 0.5 | ||
| 72 | 0.5 | ||
| Fifth | 5 | 94 | 0.5 |
| 55 | 0.5 | ||
| 72 | 0.5 | ||
| Sixth | - | 72 | 10 |
| Seventh | - | Cooling to 25 | - |
3.1. Tool
SCID: It is a structural diagnostic interview for assessing axis I psychiatric disorder based on DSM criteria. Its Persian version was equivalent to cross-cultures (22).
4. Results
The bipolar group consisted of 102 patients (74 females and 28 males) with an average age of 36.59 ± 11.35 years compared with the control group consisting of 102 healthy individuals (62 females and 40 males) with an average age of 39.22 ± 9.59 years. There were no significant differences in age and gender between the case and control groups (P = 0.076 and P = 0.075, respectively).
The genotype distribution of -511 IL-1β polymorphisms in the control group was in the Hardy-Weinberg equilibrium (P = 0.51). The frequencies of three genotypes (CC, CT, and TT) and two alleles (C and T) in each group can be seen in Table 2. There were no significant differences between genotypic frequency in bipolar and control groups (P = 1.00 for CC frequency, P = 0.261 for CT frequency, P = 0.142 for TT frequency). Also Comparing allele C frequency with allele T revealed no significant difference between two groups (P = 0.420). Besides, there were no significant differences between the two subgroups of bipolar patients (with and without suicidal ideation (P = 0.829) and with and without the psychotic feature (P = 0.218)), as shown in Table 3.
Genotypic and Allelic Frequencies of IL-1β (-511) in BP-1 and Control Groups
| IL-1β (-511) | BP Patients, No. (%) | Controls, No. (%) | P-Value | OR | Confidence Interval 95% |
|---|---|---|---|---|---|
| Genotypes | |||||
| CC | 37 (36.3) | 37 (36.3) | 1.00 | 1.00 | 0.565 - 1.770 |
| CT | 43 (42.2) | 51 (50.0) | 0.261 | 0.729 | 0.420 - 1.266 |
| TT | 22 (21.6) | 14 (13.7) | 0.142 | 1.729 | 0.829 - 3.606 |
| Alleles | |||||
| C | 117 (57.4) | 125 (61.3) | 0.420 | 0.850 | 0.572 - 1.262 |
| T | 87 (42.6) | 79 (38.7) | 0.420 | 1.177 | 0.792 - 1.747 |
Comparing Allele Frequencies Based on History of Psychosis and Suicide in the Case Group
| Conditions | Alleles | P-Value | OR | 95% Confidence Interval | |
|---|---|---|---|---|---|
| C | T | ||||
| Suicide with suicidal idea | 36 (30.8) | 28 (32.2) | 0.829 | 0.937 | 0.515 - 1.701 |
| Suicide without suicidal idea | 81 (69.2) | 59 (67.8) | |||
| Psychosis with psychotic feature | 81 (69.2) | 67 (77.0) | 0.218 | 0.672 | 0.356 - 1.268 |
| Psychosis without psychotic feature | 36 (30.8) | 20 (23.0) | |||
5. Discussion
In recent years, an increasing number of studies have suggested a crucial role for the immune system, especially pro-inflammatory cytokines such as IL-1, in the psychopathogenesis of bipolar disorder. The elevated level of pro-inflammatory cytokines in bipolar disorder was proven in several studies (11). However, it still remains unknown wheatear inflammation plays a causal role in the bipolar formation or it per se results from bipolar underlying psychopathogenesis. Among the hypotheses addressing this matter, some variations in encoding immune-related genes are proposed, which eventually may result in the dysregulation of the inflammatory cascade. Nonetheless, relatively a few studies focused on the role of genes involved in encoding immunologic components such as pro-inflammatory cytokines in bipolar pathogenesis. Further, the results of different studies in this field are controversial.
In our study, there were no significant differences in genotypic and allelic frequencies of IL-1β polymorphism in locus -511 between the bipolar and control groups, and there were either no significant differences in allelic frequencies of IL-1β polymorphism in this given locus, -511, between psychotic and non-psychotic and between suicidal and non-suicidal bipolar individuals. One study by Talaei et al. (21), which was as a pilot study for the present study, assessing and comparing the polymorphism of IL-1 cluster in four loci (-511 IL-1β, +3954 IL-1β, -889 IL-1α, and IL-1RN) between bipolar and control groups, showed significant differences in the CC genotype frequency (P = 0.04) and C/T allelic frequency (P = 0.02) just in one locus (-511 IL-1β) between 48 bipolar patients and 47 healthy individuals. Another study by Papiol et al. (12) reported an association between -511 IL-1β C/T polymorphism and gray matter deficits in the whole brain and left DLPFC of bipolar patients. Papiol et al. (13) in 2004 indicated a difference close to statistical significance for the excess of allele C in -511 C/T polymorphism (P = 0.051) in patients with schizophrenia compared to a control group whereas there were no significant differences in genotypic or allelic frequencies between bipolar patients and control groups. In line with a previous study, Kim et al. (19) reported significant differences in the allelic frequency and genotypic distribution of IL-1 receptor antagonist (IL-1RA) gene polymorphism when comparing schizophrenic patients with a control group while there were no significant differences between bipolar patients and the control group. In contrast, Rafiei et al. (18) reported a significant difference in IL-1RA gene (IL1RN) polymorphism, which plays a major role in the regulation of IL-1α and IL-1β activities, between bipolar patients and the control group, which suggested a positive correlation between the variable number of tandem repeats (VNTR) polymorphism in IL1RN and bipolar patients. Middle et al. (20) compared -308 promoter polymorphism of tumor necrosis factor-alpha (TNF-α) as a pro-inflammatory cytokine between a female control group and female bipolar patients with and without psychosis but did not support the impact of this given polymorphism on bipolar pathogenesis. In another study, the role of another pro-inflammatory cytokine, Interferon-gamma (IFN-γ), was assessed in bipolar development by comparing the IFN-γ +874A/T polymorphism between the bipolar and control groups, which suggested a possible association between the T allele and the elevated risk of bipolar development (23). Liu et al.’s study (24) indicated that the plasma levels of sCD4, sCD8, and IL-1RA significantly elevated in the acute phase of mania before pharmacotherapy while just sCD8 and IL-1RA remained different in remitted patients. In contrast, a systematic review and meta-analysis in 2013 by Munkholm et al. (25) showed no significant differences between bipolar and control groups in the serum levels of some cytokine elements such as IL-1, IL-1β, IL-1RA, and IFN-γ, while it indicated a significantly higher level of some other immune components such as TNF-α, soluble tumor necrosis factor receptor type 1 (sTNFR1), and IL-4. Another study by Rao et al. (26) suggested the role of neuroinflammation, especially IL-R cascade, in the frontal cortex of bipolar patients. Soderlund et al. (27) showed the significantly elevated level of IL-1β in patients suffering from one or more episodes of mania or hypomania in the last year when compared to patients without recent mentioned episodes, suggesting an alteration in brain cytokines which could be related to the recent episode of mania or hypomania in bipolar patients.
Finally, these controversial reports may result from heterogeneity in the methodological assessment and sample sizes of different studies, overlapping the symptoms of bipolar disorder with other psychiatric disorders, which may lead to some difficulty in its clinical diagnosis all over the world and could implicate on distinct biological origins of some different symptoms considered in overall as bipolar disorder.
5.1. Conclusions
The present study, in contrast with a previous pilot one, found no association between bipolar disorder and genotypic or allelic frequencies of -511 IL-1β polymorphism. No association was also found between the allelic frequency of -511 IL-1β polymorphism and two different subgroups, psychotic/non-psychotic and suicidal/non-suicidal, in bipolar individuals. Further studies focusing on bipolar endophenotypes are warranted.
References
-
1.
Merikangas KR, Jin R, He JP, Kessler RC, Lee S, Sampson NA, et al. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry. 2011;68(3):241-51. [PubMed ID: 21383262]. [PubMed Central ID: PMC3486639]. https://doi.org/10.1001/archgenpsychiatry.2011.12.
-
2.
WHO. The world health report 2002: reducing risks, promoting healthy life. 2002.
-
3.
Calabrese JR. Overview of patient care issues and treatment in bipolar spectrum and bipolar II disorder. J Clin Psychiatry. 2008;69(6). e18. [PubMed ID: 18683993]. https://doi.org/10.4088/jcp.0608e18.
-
4.
Burton PR, Clayton DG, Cardon LR, Craddock N, Deloukas P, Duncanson A, et al. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447(7145):661-78.
-
5.
Miller CL, Murakami P, Ruczinski I, Ross RG, Sinkus M, Sullivan B, et al. Two complex genotypes relevant to the kynurenine pathway and melanotropin function show association with schizophrenia and bipolar disorder. Schizophr Res. 2009;113(2-3):259-67. [PubMed ID: 19502010]. [PubMed Central ID: PMC2855687]. https://doi.org/10.1016/j.schres.2009.05.014.
-
6.
Drago A, Crisafulli C, Calabro M, Serretti A. Enrichment pathway analysis. The inflammatory genetic background in Bipolar Disorder. J Affect Disord. 2015;179:88-94. [PubMed ID: 25855618]. https://doi.org/10.1016/j.jad.2015.03.032.
-
7.
Rosenblat JD, McIntyre RS. Bipolar Disorder and Inflammation. Psychiatr Clin North Am. 2016;39(1):125-37. [PubMed ID: 26876323]. https://doi.org/10.1016/j.psc.2015.09.006.
-
8.
Stertz L, Magalhaes PV, Kapczinski F. Is bipolar disorder an inflammatory condition? The relevance of microglial activation. Curr Opin Psychiatry. 2013;26(1):19-26. [PubMed ID: 23196997]. https://doi.org/10.1097/YCO.0b013e32835aa4b4.
-
9.
Clerici M, Arosio B, Mundo E, Cattaneo E, Pozzoli S, Dell'osso B, et al. Cytokine polymorphisms in the pathophysiology of mood disorders. CNS Spectr. 2009;14(8):419-25. [PubMed ID: 19890236]. https://doi.org/10.1017/s1092852900020393.
-
10.
Remlinger-Molenda A, Rybakowski J. [Neuroimmunology of bipolar affective disorder]. Psychiatr Pol. 2010;44(1):27-38. [PubMed ID: 20449978].
-
11.
Muneer A. Bipolar Disorder: Role of Inflammation and the Development of Disease Biomarkers. Psychiatry Investig. 2016;13(1):18-33. [PubMed ID: 26766943]. [PubMed Central ID: PMC4701682]. https://doi.org/10.4306/pi.2016.13.1.18.
-
12.
Papiol S, Molina V, Desco M, Rosa A, Reig S, Sanz J, et al. Gray matter deficits in bipolar disorder are associated with genetic variability at interleukin-1 beta gene (2q13). Genes Brain Behav. 2008;7(7):796-801. [PubMed ID: 19125864]. https://doi.org/10.1111/j.1601-183X.2008.00421.x.
-
13.
Papiol S, Rosa A, Gutierrez B, Martin B, Salgado P, Catalan R, et al. Interleukin-1 cluster is associated with genetic risk for schizophrenia and bipolar disorder. J Med Genet. 2004;41(3):219-23. [PubMed ID: 14985387]. [PubMed Central ID: PMC1735684]. https://doi.org/10.1136/jmg.2003.012914.
-
14.
Goldstein BI, Kemp DE, Soczynska JK, McIntyre RS. Inflammation and the phenomenology, pathophysiology, comorbidity, and treatment of bipolar disorder: a systematic review of the literature. J Clin Psychiatry. 2009;70(8):1078-90. [PubMed ID: 19497250]. https://doi.org/10.4088/JCP.08r04505.
-
15.
Hope S, Dieset I, Agartz I, Steen NE, Ueland T, Melle I, et al. Affective symptoms are associated with markers of inflammation and immune activation in bipolar disorders but not in schizophrenia. J Psychiatr Res. 2011;45(12):1608-16. [PubMed ID: 21889167]. https://doi.org/10.1016/j.jpsychires.2011.08.003.
-
16.
Tsai SY, Chung KH, Wu JY, Kuo CJ, Lee HC, Huang SH. Inflammatory markers and their relationships with leptin and insulin from acute mania to full remission in bipolar disorder. J Affect Disord. 2012;136(1-2):110-6. [PubMed ID: 21962564]. https://doi.org/10.1016/j.jad.2011.08.022.
-
17.
Tsai SY, Chung KH, Huang SH, Chen PH, Lee HC, Kuo CJ. Persistent inflammation and its relationship to leptin and insulin in phases of bipolar disorder from acute depression to full remission. Bipolar Disord. 2014;16(8):800-8. [PubMed ID: 25130211]. https://doi.org/10.1111/bdi.12240.
-
18.
Rafiei A, Hosseini SH, Taheri M, Hosseni-khah Z, Hajilooi M, Mazaheri Z. Influence of IL-1RN intron 2 variable number of tandem repeats (VNTR) polymorphism on bipolar disorder. Neuropsychobiology. 2013;67(2):116-21. [PubMed ID: 23406623]. https://doi.org/10.1159/000346112.
-
19.
Kim SJ, Lee HJ, Koo HG, Kim JW, Song JY, Kim MK, et al. Impact of IL-1 receptor antagonist gene polymorphism on schizophrenia and bipolar disorder. Psychiatr Genet. 2004;14(3):165-7. [PubMed ID: 15318032]. https://doi.org/10.1097/00041444-200409000-00009.
-
20.
Middle F, Jones I, Robertson E, Lendon C, Craddock N. Tumour necrosis factor alpha and bipolar affective puerperal psychosis. Psychiatr Genet. 2000;10(4):195-8. [PubMed ID: 11324946]. https://doi.org/10.1097/00041444-200010040-00008.
-
21.
Talaei A, Tavakkol Afshari J, Fayyazi Bordbar MR, Pouryousof H, Faridhosseini F, Saghebi A, et al. A Study on the Association of Interleukin-1 Cluster with Genetic Risk in Bipolar I Disorder in Iranian Patients: A Case-control Study. Iran J Allergy Asthma Immunol. 2016;15(6):466-75. [PubMed ID: 28129679].
-
22.
Shooshtari MH, Davari-Ashtiani R, Shahrivar Z, Shabani A, Semnani Y, Kaviani H, et al. Structured clinical interview for DSM-IV (SCID Persian translation and cultural adaptation). Iran J Psychiatry. 2007;2(1):46-8.
-
23.
Yoon HK, Kim YK. The T allele of the interferon-gamma +874A/T polymorphism is associated with bipolar disorder. Nord J Psychiatry. 2012;66(1):14-8. [PubMed ID: 21728784]. https://doi.org/10.3109/08039488.2011.593045.
-
24.
Liu HC, Yang YY, Chou YM, Chen KP, Shen WW, Leu SJ. Immunologic variables in acute mania of bipolar disorder. J Neuroimmunol. 2004;150(1-2):116-22. [PubMed ID: 15081255]. https://doi.org/10.1016/j.jneuroim.2004.01.006.
-
25.
Munkholm K, Brauner JV, Kessing LV, Vinberg M. Cytokines in bipolar disorder vs. healthy control subjects: a systematic review and meta-analysis. J Psychiatr Res. 2013;47(9):1119-33. [PubMed ID: 23768870]. https://doi.org/10.1016/j.jpsychires.2013.05.018.
-
26.
Rao JS, Harry GJ, Rapoport SI, Kim HW. Increased excitotoxicity and neuroinflammatory markers in postmortem frontal cortex from bipolar disorder patients. Mol Psychiatry. 2010;15(4):384-92. [PubMed ID: 19488045]. [PubMed Central ID: PMC2844920]. https://doi.org/10.1038/mp.2009.47.
-
27.
Soderlund J, Olsson SK, Samuelsson M, Walther-Jallow L, Johansson C, Erhardt S, et al. Elevation of cerebrospinal fluid interleukin-1ss in bipolar disorder. J Psychiatry Neurosci. 2011;36(2):114-8. [PubMed ID: 21138659]. [PubMed Central ID: PMC3044194]. https://doi.org/10.1503/jpn.100080.