Abstract
Background:
The prevalence of drug-resistant Staphylococcus aureus is increasing alarmingly, limiting treatment options.Objectives:
This study was conducted to investigate the minimum inhibitory concentration (MIC) of glycopeptides against multidrug-resistant (MDR) and extensively drug-resistant (XDR) S. aureus isolates from patients with skin infections.Methods:
In this study, S. aureus isolates were collected from outpatients with skin infections (n = 250) during 2019 - 2022. The isolates were identified using routine microbiological and biochemical tests. Susceptibility to ten categories of antibacterial agents was assessed using the Kirby-Bauer method according to the Clinical and Laboratory Standards Institute M100 guidelines (2021). The MIC of glycopeptides was determined using the broth microdilution test.Results:
Among methicillin-resistant S. aureus isolates (40.8%), the majority were from patients with impetigo (53.92%). The highest resistance rate was observed against penicillin (79.41%) and doxycycline (73.52%). Linezolid showed significant inhibitory properties against XDR (91%) and MDR (97%) S. aureus isolates (P = 0.01). The MIC of oritavancin that inhibited the growth of 90% of the MDR isolates (MIC90) was 2 µg/mL, which was eight times less than that of vancomycin (MIC90 = 16 µg/mL) and 16 times lower than that of teicoplanin (MIC90 = 32 µg/mL) in a manner that 91% of MDR isolates from impetigo were eliminated at concentrations 2 µg/mL. Oritavancin inhibited the growth of 54.5% of XDR isolates at MIC concentrations of ≥8 µg/mL.Conclusions:
Considering the strong antibacterial activity of linezolid against MDR S. aureus isolates, this antibiotic can effectively treat skin infections caused by S. aureus and prevent the development of resistance to other antibiotics. In addition, considering the great inhibitory properties of oritavancin against MDR S. aureus strains, the efficacy of this agent for treating skin infections, particularly impetigo, should be investigated.Keywords
Staphylococcus aureus Drug Resistance Skin Infection Oritavancin
1. Background
Staphylococcus aureus is a major cause of skin infections that may present in the form of boils, folliculitis, impetigo, cellulitis, invasive soft tissue infections, foot ulcer, and furuncle. In skin infections, bacterial colonization in the skin due to skin barrier disruption leads to the expression of cytokines, which ultimately aggravates the symptoms (1-3). Infections caused by S. aureus pose a significant challenge to treating wounds and damaged tissues. Such infections are of great clinical importance because of S. aureus pathogenicity and its transmission and antibiotic resistance capacity. Therefore, timely and appropriate antibiotic therapy is essential for managing S. aureus infections.
Since the emergence and spread of multi-drug resistant (MDR) S. aureus strains, including methicillin-resistant S. aureus (MRSA) in 1961, the World Health Organization has classified this bacterium as a serious threat to the control of various infections, including skin infections. The prevalence of infection with extensively drug-resistant (XDR) S. aureus is also increasing. MDR is defined as non-susceptibility to ≥1 antimicrobial agent in ≥3 antimicrobial categories, and XDR is defined as non-susceptibility to ≥1 antimicrobial agent in all the antimicrobial categories, except in ≤2. This bacterium can acquire antibiotic resistance through several biochemical pathways, including modifying and destroying antibiotic molecules, reducing antibiotic penetration, or altering the bacterial target site (4, 5). Resistant skin infections are usually treated with beta-lactams, especially cephalosporins, such as cefazolin or glycopeptides (6). Vancomycin and teicoplanin were the first glycopeptides clinically used in 1955 and 1984, respectively. Oritavancin diphosphate (LY333328) is a semi-synthetic lipoglycopeptide introduced in 2015 with broad antibacterial activity against Gram-positive bacteria, including penicillin-resistant streptococci and coagulase-positive staphylococci. This agent acts on the peptide backbone by binding to the D-alanyl-D-alanine terminal of the peptidoglycan chain of Gram-positive bacteria, thereby blocking transglycosylation during peptidoglycan synthesis and preventing the formation of the bacterial cell wall. In addition, oritavancin can bind to the cytoplasmic membrane through its alkyl side chain, which in turn increases binding affinity to peptidoglycan residues and activity against vancomycin-resistant enterococci and vancomycin-resistant S. aureus (7-9).
2. Objectives
Considering the high prevalence of skin infections caused by S. aureus and the high rate of resistance to various antibacterial agents, this study aimed to evaluate the incidence of MDR, XDR isolates, and the minimum inhibitory concentration (MIC) of glycopeptides, particularly oritavancin, against drug-resistant S. aureus isolates from outpatients with skin infections.
3. Methods
3.1. Study Design and Patients’ Characteristics
In this prospective cross-sectional study, skin samples were taken from 250 outpatients (age range 11 – 72 years mean ± SD 43 ± 9.1) referred to seven Golestan Province (northern Iran) hospitals during 2019 - 2022. The samples were selected randomly via the convenience sampling method by dermatologists or infectious disease specialists. The inclusion criterion was clinical signs of skin infection and no antibiotic use in the last three months. The sample size was determined with a 95% confidence level using the formula below, where P1 is the number of patients referred to the infectious disease ward and P2 represents the number of patients with a positive MRSA test (α = 0.05, β = 0.10).
A questionnaire was prepared to collect characteristics of patients, including gender, age, type of skin infection, and the referral season. Patients with autoimmune diseases and those under ten (male and female) were excluded from this study.
3.2. Identification of Isolates
To identify and confirm the bacterial agents, skin swabs were first placed in tryptone soya broth (Merck, Germany) and then transferred to the medical diagnosis laboratory of the Infectious Diseases Research Center of Shahid Beheshti University. Then, the samples were inoculated onto chocolate agar, Columbia agar with 5% horse blood, and MacConkey agar. After 48 hours of incubation at 37°C, S. aureus strains were identified by examining mannitol-positive colonies and analyzing colony morphology, Gram staining, hemolysis, and catalase, coagulase (clumping factor), and DNase tests, and ultimately the Vitek-2 card system (Biometrics, France).
3.3. Determination of MRSA Isolates
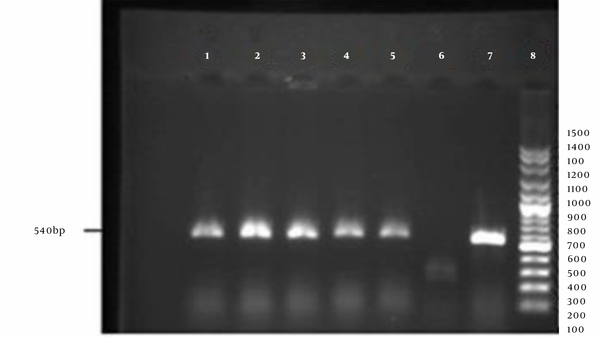
Phenotypic characterization of MRSA isolates was done by the Kirby-Bauer method using cefoxitin disks (30 µg). Detection of a growth inhibition zone with a diameter of ≤21 mm confirmed the presence of MRSA isolates. For a definite identification of MRSA strains, after DNA extraction using a commercial kit (SinaClon, Iran) according to the manufacturer's instructions, polymerase chain reaction (PCR) was done using the following primers (designed by Oligo 5 software) that are specific for the mecA gene (methicillin resistance gene): forward: 5'- AGTTCTGCAGTACCGGATTTGC -3' and reveres: 5'-AAAATCGATGGTAAAGGTTGGC-3'. Standard strains of S. aureus ATCC 33591 and S. aureus ATCC 25923 were considered positive and negative controls, respectively. The PCR reaction was carried out in a final volume of 25 µL consisting of 1 µL DNA sample, 1 µL of each primer, 12 µL of 2X Master Mix (containing 20 µM dNTP and 1.5 µM MgCl2), and 11 µL of distilled water. The reaction was performed in a thermocycler (Eppendorf, Germany) with the following cycling conditions: initial denaturation at 95°C for 5 minutes, 30 cycles of denaturation at 94°C for 15 seconds, annealing at 61°C for 1 minute, extension at 72°C for 1 minute, and final extension at 72°C for 5 minutes. The resulting PCR products were then electrophoresed on 1.5% agarose gel. The detection of 540 bp fragments confirmed the presence of MRSA isolates.
3.4. Antibacterial Susceptibility Testing
Antibiotic susceptibility test was performed on Mueller- Hinton agar (Merck, Germany) by the disk diffusion method (Kerby-Bauer method) using the following antibiotic disks: linezolid (10 µg), doxycycline (10 µg), amikacin (30 µg), daptomycin (2 µg), ciprofloxacin (5 µg), cefuroxime (30 µg), cefazolin (30 µg), clindamycin (2 µg), penicillin (10 units), and azithromycin (15 µg). All antibiotic discs were purchased from Padtan Teb Co. (Iran) except for linezolid (purchased from Mast Group, UK). After 16 - 18 hours of incubation at 37°C, the results were interpreted by measuring the diameter of the growth inhibition zone according to the Clinical & Laboratory Standards Institute (CLSI) standard guidelines (2021) (10).
3.5. Determination of Glycopeptides Minimum Inhibitory Concentration(s)
The MIC of three glycopeptides, including teicoplanin, vancomycin, and oritavancin, against the MRSA isolates was determined using the broth microdilution method according to the CLSI M100 guidelines (10). To prepare an antibacterial suspension, necessary amounts of vancomycin and teicoplanin powder (Sigma-Aldrich, USA) were inoculated into water and oritavancin powder into 0.002% polyphosphate in water. The initial concentration of each antibiotic was inoculated into wells of a 96-well microplate containing Mueller Hinton broth (Merck, Germany) and 2% salt. After preparing serial dilutions in the range of 0.06 - 64 µg/mL and inoculating the bacterial suspension at a final concentration of 1.5 × 105 colony-forming units, the microplate was incubated at 37°C for 20 - 24 hours. A well containing the medium and antibiotic stock and another containing the medium with bacterial suspension were considered negative and positive controls, respectively. The MIC was determined by measuring the absorbance at 560 nm using an ELISA reader (10). Staphylococcus aureus ATCC 25923 and S. aureus ATCC 29213 were used as the control strains in the Kirby-Bauer and broth microdilution methods, respectively.
3.6. Statistical Analysis
Data were presented as frequency tables, graphs, and numerical indices. All data were analyzed using SPSS (version 23), and intergroup comparisons were done using the chi-square test at a significance level of <0.05.
4. Results
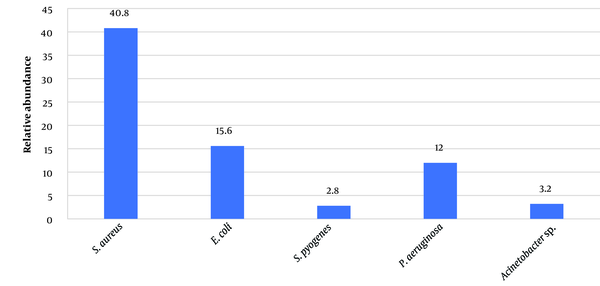
The most common and least common isolates were MRSA (102,40.80%) and Staphylococcus pyogenes (2.80%), respectively (Figure 1). Based on the phenotypic and molecular investigations (Figure 2), the frequency of MRSA isolates was significantly higher in samples collected from impetigo (53.92%) (P = 0.03) and in the summer season (44.11%) (P = 0.02) (Table 1).
The relative frequency of bacterial strains isolated from patients with skin infections
PCR amplification of the mecA gene. 1-5: Positive samples; 6: Negative control; 7: Positive control; 8: DNA ladder
Characteristics of the Study Population and Frequency of MDR and XDR Staphylococcus aureus Isolates a
| Variables | MDR (n = 91) | XDR (n = 11) | χ2 | P-Value |
|---|---|---|---|---|
| Age, y | 0.07 | |||
| 11 - 17 | 13 (14.28) | 0 (0 ) | 0.17 | |
| 18 - 44 | 63 (69.23) | 7 (63.63) | 0.21 | |
| ≥45 | 15 (16.48) | 4 (36.36) | 0.29 | |
| Gender | 0.06 | |||
| Female | 71 (78.02) | 4 (36.36) | 0.49 | |
| Male | 20 (21.97) | 7 (63.63) | 0.34 | |
| Season | 0.02 b | |||
| Spring | 33 (36.26) | 5 (45.45) | 0.37 | |
| Summer | 41 (45.05) | 4 (36.36) | 0.32 | |
| Autumn | 11 (12.08) | 2 (18.18) | 0.21 | |
| Winter | 6 (6.59) | 0 (0 ) | 0.11 | |
| Skin infection | 0.03 b | |||
| Foot ulcers | 35 (38.46) | 6 (54.54) | 0.41 | |
| Impetigo | 51 (56.04) | 4 (36.36) | 0.45 | |
| Cellulitis | 5 (5.49) | 1 (9.09) | 0.30 |
Among MRSA isolates, 89.21% and 10.78% were MDR and XDR, respectively. In the Kirby-Bauer disk diffusion method, the highest and lowest drug resistance was related to penicillin (79.41%) and linezolid (2.94%). Cefazolin and cefuroxime were in second place in terms of antibacterial potency. Linezolid exhibited significant inhibitory effects against 98% of the isolates, especially S. aureus isolates, from foot ulcers in patients with diabetes. (Table 2). Moreover, linezolid inhibited the growth of 91% of XDR and 97% of MDR isolates (P = 0.01).
Frequency of Resistance of MRSA Isolates from Skin Infections Against Different Antibiotics a
| Antibiotic | Foot ulcers (n = 41), Resistance | Impetigo (n = 55), Resistance | Cellulitis (n = 6), Resistance |
|---|---|---|---|
| Linezolid | 2 (4.87) | 0 (0 ) | 1 (16.67) |
| Daptomycin | 14 (34.14) | 9 (16.36) | 5 (83.33) |
| Clindamycin | 29 (70.73) | 33 (60 ) | 6 (100 ) |
| Ciprofloxacin | 17 (41.46) | 11 (20 ) | 3 (50 ) |
| Cefuroxime | 14 (34.14) | 7 (12.72 ) | 3 (50 ) |
| Azithromycin | 20 (48.78) | 13 (23.63) | 4 (66.67) |
| Doxycycline | 31 (75.60) | 39 (70.90) | 5 (83.33) |
| Penicillin | 35 (85.36) | 40 (72.72) | 6 (100 ) |
| Cefazolin | 15 (36.58) | 5 (9.09) | 2 (33.33) |
| Amikacin | 21 (51.21) | 12 (21.81) | 5 (83.33) |
At MIC ≥2 µg/mL, oritavancin inhibited the growth of 81.82% of MDR and, at MIC ≥8 µg/mL, 54.5 % of XDR isolates in a dose-dependent manner. In the case of glycopeptides, the lowest concentration of oritavancin that inhibited the growth of 90% of the MDR isolates (MIC90) was 2 µg/mL, which was eight times less than that of vancomycin (MIC90 = 16 µg/mL) and 16 times lower than that of teicoplanin (MIC90 = 32 µg/mL) (Tables 3 and 4).
MICs Average of Different Glycopeptides Against MDR Staphylococcus aureus Isolates from Skin Infections
| Glycopeptides | MIC | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration | 64 | 32 | 16 | 8 | 4 | 2 | 1 | 0.5 | 0.25 | 0.125 | 0.06 |
| Vancomycin | - | - | √ | - | - | - | - | - | - | - | - |
| Teicoplanin | - | √ | - | - | - | - | - | - | - | - | - |
| Oritavancin | - | - | - | - | - | √ | - | - | - | - | - |
Summary of the Activity of Different Antibiotics Against XDR Staphylococcus aureus Isolates
| Antibiotics XDR (11) | MIC90 (µg/mL) | MIC50 (µg/mL) | Range | Percentage of Resistance |
|---|---|---|---|---|
| Oritavancin | ≥8 | 2 | 1 - 8 | 45.45 |
| Vancomycin | ≥16 | 4 | 4 - 32 | 54.55 |
| Teicoplanin | >32 | 8 | 4 to 64 | 63.63 |
In the present study, the MIC average of oritavancin against MDR isolates from impetigo has been determined at 1.85 µL/mL, in which most growth fluctuations were observed in the density of 0.25 and 0.125 µL/mL. At the same time, this mean was 1.93 and 2.00 µL/mL for foot ulcer and cellulite, respectively. Most oritavancin-resistant MDR S. aureus isolates were from the specimens of cellulite (3 out of 6). In total, 15 cases (14.70%) of MDR S. aureus isolates were resistant to oritavancin, whereas (47, 85.45%) of MDR isolates from impetigo cases were sensitive to oritavancin. Oritavancin also showed favorable antibacterial activity against 60% of vancomycin-resistant XDR strains, 85.71% of all teicoplanin-resistant XDR strains, and 91% of MDR/XDR strains isolated from impetigo.
The frequency of vancomycin-resistance and teicoplanin-resistance among MDR S. aureus isolates was 26.47% and 34.31%, respectively. The average MIC of vancomycin and teicoplanin in order against MDR isolates from impetigo was determined at 14.40 and 31.62 µL/mL.
5. Discussion
It is well-documented that MRSA is one of the most important causes of skin infections in many parts of the world. However, different bacterial pathogens may cause skin infections, highlighting the role of environmental conditions, personal hygiene standards, age, infection site, and even season (11, 12). In the present study, more than half of S. aureus isolates from impetigo, and 40.8% of those from all skin infections were MRSA. These findings are in line with the findings of a previous study in Iran (13). Similarly, two other studies in Iran reported that more than half of all isolates from skin infections were MRSA (14, 15). In a recent study, 48% of S. aureus isolates from wounds, secretions, and blood samples were MRSA, of which 61% were MDR (6), which is less than the frequency of MDR strains in our study (89%), Based on our results, 10.8% of MRSA isolate were XDR. In contrast, in Pakistan (2020), 20%, and in Iran, a Middle East country (2022), 48% of the isolates were identified as XDR (16, 17).
Excessive use of antibiotics has significantly increased the prevalence of bacterial skin infections, creating a serious health challenge (18, 19).
In this study, among MDR S. aureus isolates, the highest antibiotic resistance was against penicillin (79.41%) and doxycycline (73.52%), which is consistent with previous reports (20). As an oxazolidinone, linezolid generally has a high antibacterial potential. In this study, 97% of MDR and 91% of XDR S. aureus isolates were sensitive to linezolid. Among MRSA isolates, 78.44% and 76.48% were sensitive to cephalosporines cefazolin and cefuroxime, respectively. However, it should be noted that glycopeptides are usually considered the first and second line of treatment against infections caused by Gram-positive bacteria (21, 22).
In our study, vancomycin and teicoplanin showed moderate antibacterial activity, and 26.47% and 34.31% of isolates were resistant, respectively. In a study in Iran in 2016, 23.3% of S. aureus isolates from abscesses and wounds showed resistance to vancomycin (22). However, a year later, another study in Iran reported that all S. aureus isolates were sensitive to vancomycin (23). The higher rate of vancomycin-resistance among S. aureus isolates in the present study could be attributed to the difference in the study time, the floods in 2019 in our study location, the high prevalence of impetigo in flood victims and the difference in the type of samples.
Compared to other glycopeptides, oritavancin can exert rapid and dose-dependent bactericidal activity (24). In this study, 85.30% of MDR isolates were sensitive to oritavancin, and this agent showed the highest inhibitory effect on MRSA isolates at a concentration of 1 - 2 µg/mL. It also showed an inhibitory effect on XDR S. aureus isolates at concentrations of 8 µg/mL and higher. In this regard, previous studies in Canada and the United States also reported the high efficiency of oritavancin in treating acute skin infections and in vivo pharmacokinetic models (25, 26). A study in 2021 and a meta-analysis study (2022) demonstrated the efficacy of oritavancin therapy compared with other glycopeptides for controlling skin infections in hospitalized patients, which could prevent recurrences with fewer side effects (27, 28). Various trials on animal models and patients with complex skin infections have also demonstrated that oritavancin can be the antibiotic of choice because of its shorter treatment duration, safety in children, and fewer side effects (29-31).
A limitation of this study was the small patient sample size and, most importantly, the COVID-19 pandemic that prevented us from comparing clinical features with the microbiological results. However, this study had strengths that can facilitate the development of skin infection treatment guidelines.
5.1. Conclusions
Our results highlighted the alarmingly high rate of resistance to antibiotics among S. aureus isolates from patients with skin infections. In line with previous studies, S. aureus was confirmed as the most common cause of skin infections in our study. Considering the great inhibitory properties of oritavancin against MDR S. aureus strains, especially those isolated from impetigo patients, the efficacy of this antibiotic for treating skin infections, particularly impetigo, is imperative. Moreover, oritavancin should be included in the skin sample antibiogram of medical diagnostic laboratories.
References
-
1.
Hsieh YC, Lin YC, Huang YC. Vancomycin, teicoplanin, daptomycin, and linezolid MIC creep in methicillin-resistant Staphylococcus aureus is associated with clonality. Medicine (Baltimore). 2016;95(41). e5060. [PubMed ID: 27741120]. [PubMed Central ID: PMC5072947]. https://doi.org/10.1097/MD.0000000000005060.
-
2.
Sina H, Ahoyo TA, Moussaoui W, Keller D, Bankole HS, Barogui Y, et al. Variability of antibiotic susceptibility and toxin production of Staphylococcus aureus strains isolated from skin, soft tissue, and bone related infections. BMC Microbiol. 2013;13:188. [PubMed ID: 23924370]. [PubMed Central ID: PMC3750628]. https://doi.org/10.1186/1471-2180-13-188.
-
3.
Chatterjee SS, Otto M. Improved understanding of factors driving methicillin-resistant Staphylococcus aureus epidemic waves. Clin Epidemiol. 2013;5:205-17. [PubMed ID: 23861600]. [PubMed Central ID: PMC3707418]. https://doi.org/10.2147/CLEP.S37071.
-
4.
Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268-81. [PubMed ID: 21793988]. https://doi.org/10.1111/j.1469-0691.2011.03570.x.
-
5.
Dar JA, Thoker MA, Khan JA, Ali A, Khan MA, Rizwan M, et al. Molecular epidemiology of clinical and carrier strains of methicillin resistant Staphylococcus aureus (MRSA) in the hospital settings of north India. Ann Clin Microbiol Antimicrob. 2006;5:22. [PubMed ID: 16972997]. [PubMed Central ID: PMC1592298]. https://doi.org/10.1186/1476-0711-5-22.
-
6.
Balli EP, Venetis CA, Miyakis S. Systematic review and meta-analysis of linezolid versus daptomycin for treatment of vancomycin-resistant enterococcal bacteremia. Antimicrob Agents Chemother. 2014;58(2):734-9. [PubMed ID: 24247127]. [PubMed Central ID: PMC3910884]. https://doi.org/10.1128/AAC.01289-13.
-
7.
Schulz LT, Dworkin E, Dela-Pena J, Rose WE. Multiple-Dose Oritavancin Evaluation in a Retrospective Cohort of Patients with Complicated Infections. Pharmacotherapy. 2018;38(1):152-9. [PubMed ID: 29121395]. https://doi.org/10.1002/phar.2057.
-
8.
Sahm DF, Deane J, Bien PA, Locke JB, Zuill DE, Shaw KJ, et al. Results of the surveillance of Tedizolid activity and resistance program: in vitro susceptibility of gram-positive pathogens collected in 2011 and 2012 from the United States and Europe. Diagn Microbiol Infect Dis. 2015;81(2):112-8. [PubMed ID: 25488274]. https://doi.org/10.1016/j.diagmicrobio.2014.08.011.
-
9.
Miller WR, Murray BE, Rice LB, Arias CA. Resistance in Vancomycin-Resistant Enterococci. Infect Dis Clin North Am. 2020;34(4):751-71. [PubMed ID: 33131572]. [PubMed Central ID: PMC7640809]. https://doi.org/10.1016/j.idc.2020.08.004.
-
10.
Clinical and Laboratory Standards Institute. M100: Performance standards for antimicrobial susceptibility testing: 31st informational supplement. 31th ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2021.
-
11.
Corey GR, Loutit J, Moeck G, Wikler M, Dudley MN, O'Riordan W, et al. Single Intravenous Dose of Oritavancin for Treatment of Acute Skin and Skin Structure Infections Caused by Gram-Positive Bacteria: Summary of Safety Analysis from the Phase 3 SOLO Studies. Antimicrob Agents Chemother. 2018;62(4). [PubMed ID: 29358292]. [PubMed Central ID: PMC5914007]. https://doi.org/10.1128/AAC.01919-17.
-
12.
Tahaei M, Fozouni L, Khademi M. Molecular Investigation of Enterotoxigenic Staphylococcus aureus Isolates from Restaurant Staff. Middle East J Rehabil Health Stud. 2022;9(4). https://doi.org/10.5812/mejrh-129477.
-
13.
Sadat SS, Ahani Azari A. Frequency of Multidrug-Resistant, Extensively Drug-Resistant, and Pandrug-Resistant Phenotypes among Clinical Isolates of Staphylococcus aureus. Infect Epidemiol Microbiol. 2020;6(4):269-75. https://doi.org/10.29252/iem.6.4.269.
-
14.
Havaei SA, Ahmadpour M, Poursina F, Ruzbahani M, Assadbeigi B. [The prevalence of Panton-Valentine leukocidin gene in staphylococcus aureus isolated from Alzahra Hospital, Isfahan, Iran]. J Isfahan Med Sch. 2015;32(315):2217-25. Persian.
-
15.
Motamedi H, Rahmat Abadi SS, Moosavian SM, Torabi M. The Association of Panton-Valentine leukocidin and mecA Genes in Methicillin-Resistant Staphylococcus aureus Isolates from Patients Referred to Educational Hospitals in Ahvaz, Iran. Jundishapur J Microbiol. 2015;8(8). e22021. [PubMed ID: 26468365]. [PubMed Central ID: PMC4601108]. https://doi.org/10.5812/jjm.22021v2.
-
16.
Sabir N, Hussain W, Ahmed A, Zaman G, Ali Mirza I, Ikram A. Burden of Multi-Drug Resistant, Extensively-Drug Resistant and Pan-Drug Resistant Superbugs Isolated from Various Indoor Microbiological Specimens at Tertiary Care Centers Rawalpindi. Pak Armed Forces Med. 2020;70(1):79-85.
-
17.
Fozouni L, Pishdad Z, Malekpour Kolbadinezhad S. The Potency of Tedizolid, Linezolid, and Vancomycin Against Extensively Drug-Resistant Staphylococcus aureus Clinical Isolates. Zahedan J Res Med Sci. 2022;24(4). https://doi.org/10.5812/zjrms-114238.
-
18.
Morrissey I, Seifert H, Canton R, Nordmann P, Stefani S, Macgowan A, et al. Activity of oritavancin against methicillin-resistant staphylococci, vancomycin-resistant enterococci and beta-haemolytic streptococci collected from western European countries in 2011. J Antimicrob Chemother. 2013;68(1):164-7. [PubMed ID: 22941898]. https://doi.org/10.1093/jac/dks344.
-
19.
Mendes RE, Castanheira M, Farrell DJ, Flamm RK, Sader HS, Jones RN. Prevalence of macrolide-lincosamide resistance and multidrug resistance phenotypes in streptococcal isolates causing infections in European hospitals: Evaluation of the in vitro activity of oritavancin and comparator agents. J Glob Antimicrob Resist. 2017;8:28-32. [PubMed ID: 27939809]. https://doi.org/10.1016/j.jgar.2016.08.013.
-
20.
Denton M, O'Connell B, Bernard P, Jarlier V, Williams Z, Henriksen AS. The EPISA study: antimicrobial susceptibility of Staphylococcus aureus causing primary or secondary skin and soft tissue infections in the community in France, the UK and Ireland. J Antimicrob Chemother. 2008;61(3):586-8. [PubMed ID: 18222949]. https://doi.org/10.1093/jac/dkm531.
-
21.
Herrmann DJ, Peppard WJ, Ledeboer NA, Theesfeld ML, Weigelt JA, Buechel BJ. Linezolid for the treatment of drug-resistant infections. Expert Rev Anti Infect Ther. 2008;6(6):825-48. [PubMed ID: 19053895]. https://doi.org/10.1586/14787210.6.6.825.
-
22.
Esfahani F, Fozouni L, Pordeli H. A study on the antimicrobial effect of zinc oxide nanoparticles on clinical strains of Staphylococcus aureus resistant to vancomycin. Int J Mol Cell Med. 2016;6(2):693-9.
-
23.
Fagheei-Aghmiyuni Z, Khorshidi A, Soori T, Moniri R, Mousavi SGA. [Antibiotic susceptibility pattern and the prevalence of Staphylococcus aureus isolated from skin and soft tissue in Tehran Razi skin hospital (2014-15)]. Feyz Med Sci J. 2017;21(2):188-96. Persian.
-
24.
Sweeney D, Shinabarger DL, Arhin FF, Belley A, Moeck G, Pillar CM. Comparative in vitro activity of oritavancin and other agents against methicillin-susceptible and methicillin-resistant Staphylococcus aureus. Diagn Microbiol Infect Dis. 2017;87(2):121-8. [PubMed ID: 27890418]. https://doi.org/10.1016/j.diagmicrobio.2016.11.002.
-
25.
Lodise TP, Fan W, Sulham KA. Economic Impact of Oritavancin for the Treatment of Acute Bacterial Skin and Skin Structure Infections in the Emergency Department or Observation Setting: Cost Savings Associated with Avoidable Hospitalizations. Clin Ther. 2016;38(1):136-48. [PubMed ID: 26708118]. https://doi.org/10.1016/j.clinthera.2015.11.014.
-
26.
Belley A, Arhin FF, Moeck G. Evaluation of Oritavancin Dosing Strategies against Vancomycin-Resistant Enterococcus faecium Isolates with or without Reduced Susceptibility to Daptomycin in an In Vitro Pharmacokinetic/Pharmacodynamic Model. Antimicrob Agents Chemother. 2018;62(1). [PubMed ID: 29109163]. [PubMed Central ID: PMC5740372]. https://doi.org/10.1128/AAC.01873-17.
-
27.
Zhang H, Zhou W, Wang J, Cai Y. Efficacy and safety of oritavancin for the treatment of acute bacterial skin and skin-structure infections: a systematic review and meta-analysis. J Glob Antimicrob Resist. 2021;25:380-9. [PubMed ID: 33989846]. https://doi.org/10.1016/j.jgar.2021.04.013.
-
28.
Liu F, Rajabi S, Shi C, Afifirad G, Omidi N, Kouhsari E, et al. Antibacterial activity of recently approved antibiotics against methicillin-resistant Staphylococcus aureus (MRSA) strains: A systematic review and meta-analysis. Ann Clin Microbiol Antimicrob. 2022;21(1):37. [PubMed ID: 35978400]. [PubMed Central ID: PMC9382732]. https://doi.org/10.1186/s12941-022-00529-z.
-
29.
Diekema DJ, Hsueh PR, Mendes RE, Pfaller MA, Rolston KV, Sader HS, et al. The Microbiology of Bloodstream Infection: 20-Year Trends from the SENTRY Antimicrobial Surveillance Program. Antimicrob Agents Chemother. 2019;63(7). [PubMed ID: 31010862]. [PubMed Central ID: PMC6591610]. https://doi.org/10.1128/AAC.00355-19.
-
30.
Garau J, Ostermann H, Medina J, Avila M, McBride K, Blasi F, et al. Current management of patients hospitalized with complicated skin and soft tissue infections across Europe (2010-2011): assessment of clinical practice patterns and real-life effectiveness of antibiotics from the REACH study. Clin Microbiol Infect. 2013;19(9):E377-85. [PubMed ID: 23663184]. https://doi.org/10.1111/1469-0691.12235.
-
31.
Hoover RK, Krsak M, Molina KC, Shah K, Redell M. Kimyrsa, An Oritavancin-Containing Product: Clinical Study and Review of Properties. Open Forum Infect Dis. 2022;9(5):ofac090. [PubMed ID: 35392455]. [PubMed Central ID: PMC8982769]. https://doi.org/10.1093/ofid/ofac090.