Abstract
Background:
One of the important side effects in spinal cord injuries in both genders is sexual dysfunction. This study aimed at investigating histopathological changes of ovaries in the chronic phase after spinal cord injury.Methods:
Animals (adult female rats) were divided to the following groups, Control (Co), Sham (Sh), and Spinal Cord Injury (SCI); each group contained 18 rats. The spinal cord of SCI animals was transected by the bilateral laminectomy method on level T9, while the rats of the sham group were incised in the same location without any spinal cord injury. Animals were sacrified at 7th, 14th and 21st day postsurgical intervention, then the body weight, and the weight and volume of ovaries were measured. The slices were stained by hematoxylin and eosin and periodic acid Schiff methods. The histomorphometric changes of the diameter of the follicle and ovum, and the thickness of granulosa layer were measured in different kinds of follicles including, Unilaminar Primary Follicle (UPF), Multilaminar Primary Follicle (MPF), Secondary Follicle (SF), and Tertiary Follicle (TF).Results:
The results showed that the animal weights were decreased in three SCI groups (P = 0.018). In the SCI groups, the diameter of follicle and ovum and the thickness of granulosa layer were significantly decreased in different kinds of follicles (P = 0.012). Also the thickness of zona pellucida and theca interna were significantly decreased in UPF, SF, and TF in the SCI groups on 14th and 21st day (P = 0.024). The histopathologic examination revealed widespread ovarian follicle atresia in the SCI groups on the 14th and 21st day, including numerous cell debris and inflammatory cells in the antrum atretic follicles. The ovarian stroma showed edema, fibrosis, hypercellularity, and vasodilation in the SCI group, compared to the sham or control groups.Conclusions:
The histopathlogic data indicated that after spinal cord injury many histologic parameter changes occurred and hetrogenity of ovarian structure increased. These changes may be caused by dysfunction of the autonomic system and the modification in amount of the ovarian neurotransmiters.Keywords
1. Background
Sexual and reproductive health is an important quality of life component, which can have a major impact on patient satisfaction. Spinal pathology arising from trauma, deformity, and degenerative disease processes may be harmful to sexual and reproductive function (1).
Natural fertility of females is a consequence of regular and coordinated biological processes. The neurohormonal system plays a very important and vital role in regulation and stability of the reproductive system (2-4).
Sexual dysfunction is an important complication of spinal cord injury (SCI). Similar to many other complications of SCI, sexual function is also affected by the integrity of neural pathways, motor, sensory and autonomic, as well as psychological and social factors (5).
Sexual dysfunction in patients with SCI is a product of 2 major factors: the physiological changes resulting from the neurological deficit and the psycho-social consequences of the disability. Sexual disturbances in females after SCI are less understood and described than those occurring in post-SCI males (6, 7).
Recent findings have shown that in mamalian ovaries content aggregation of neuron perikaryons occurs, specially in close proximity with theca folliculi layer, interestitial glands, and vessels. Some of these neurons are sympathetic and have an important role on regulation of ovarian function (8, 9). Other evidences have shown that the ovary is innervated by external adrenergic nerves in 2 ways; superior ovarian nerve and ovarian plexus around the ovarian artery (10, 11). Previous studies have shown that superior ovarian nerve transection decresses ovarian progesterone level and effects the maturation of its steroids (12-14). Some studies stated that in females with spinal transection, Follicule stimulating hormone (FSH) level decreased and estrogen had little effect on vaginal mucosa (15). In animals with bilateral transection of ovarian nerve, decreases were observed at the intra-ovarian norepinephrine level. Also these findings suggested that hormonal regulation of the ovaries is directly controled by central nervous system via specific neuronal pathways (10-13, 15).
Other studies reported that ovarian nerves participate in differentiation of primary follicles, cosisting of FSH receptors, via neurotransmitter connection to cyclic Adenosine Monophosphate (cAMP) productive system. Therefore, as follicles are grown in parts of the ovary including higher density of neurons, they could be more rapidly regulated by gonadotropins (14). On the other hand, it has been reported that CNS includes specific cells in parapyramidal nuclei, caudal rapha, paraventricles, and lateral hypothalamus that play an effective role on ovarian function via sympathetic or vagus nerves (16). There are evidences that prove the neuro-hormonal effects on ovarian physiology, specially follicular maturation and ovulation (16).
Though ovaries in mammals are innervated by spinal nerves (T10-L2) and abdominal vagal branches, yet, progressive signaling occurs only through the spinal cord. Therefore, the SCI disconnects oncoming signaling transport from gonads to the brain, which cut off the liberation of gonadotropin from the hypothalamus and lead to hypogonadism of patients with SCI (17, 18). Although the ovaries are transmitted with both the vagus nerve and the spinal cord branches, the continuaion of usual ovarian action after SCI could be mainly due to the preservation of neuronal tissue of the spinal cord. Hence, interruptions in signaling following SCI changes ovarian physiology via inflammation and apoptosis (17, 18).
Spinal cord injury is one of the complications of medicines that effect organs of the extremities and several systems of the human body. One of the most important complications of spinal cord injury is sexual disorders in both genders that may lead to major psychological and mental problems. Findings of earlier studies and our results have demonstrated that male rats with acute and chronic spinal cord injury, showed extensive histopathological changes, including spermatogenesis arrest, decrease of seminiferous epithelial thickness, and decrease of prostate and seminal vesicle epithelium, etc. These structural changes lead to sexual dysfunctions of reproductive organs such as testis, prostate gland, seminal vesicle, and genital ducts (2, 19-21).
Although the frequency of females with spinal cord injury is low, yet, sexual dysfunction in this gender is more complicated and unclear (2, 3). Thus, findings of some reports about spinal cord transection effects on females reproductive system is impossible and there are only a few studies about the physiological and hormonal outcomes of these patients.
The goal of this research was to study the structural and histopathological alterations in ovaries following chronic spinal cord transection in wistar rats.
2. Methods
2.1. Experimental Animals
Overall, 54 adult female wistar rats (180 ± 20 g) were included in this interventional experimental study. The rats were maintained in 12:12 light and dark photoperiod, relative humidity of 45% to 55%, and temperature of 24 ± 2°C and free access to standard diet and water. All experimental protocols were approved by the Institutional animal ethics committee of Semnan University of Medical Sciences.
2.2. Spinal Cord Injury Procedure
Following a week of acclimation, the rats were randomly divided to 3 groups (n = 18 for each group): Spinal Cord Injury group (SCI, animals with their spinal cord transected), Sham group (Sh, animals only had an incision without their spinal cord transected) and Control group (Co, without incision). Animals were examined on 7th, 14th and 21st day after the procedure. In the SCI group, animals were anesthetized by administering ketamine/xylazine (80/20 mg/kg), intraperitoneally. Then, T9 vertebrate laminae were cut by bilateral laminectomy and then transverse transection of the spinal cord was made (20, 21). The rats in the sham group were incised in the same location without spinal cord transected. No intervention was implemented for the control group. The location of the surgery in the SCI and sham groups was disinfected and muscles, fascia, and skin were sutured. In the SCI group, in order to prevent probable mortality due to lower limb paralysis and numbness, special care was applied.
2.3. Animals Special Cares
The place in which the rats were kept was clean, and the tempreture was regulated. Neurogenic bladder due to spinal cord injury was evacuated regularly. In this study, handling of the bladder was achieved by the Linsenmeyer method (22). External genitalia was disinfected with 70% alchol after bladder evacuation. All animals in sham and SCI groups received 5 mg/kg of gentamicin once a day for 5 days post-surgery. For a possible bladder infection control, 2.5 mg/kg of ketoprofen once a day for 2 days was administered to reduce post-surgical pain (20, 22, 23). To aviod autophagia, heterophagia and following mortality rate, paralytic extremities care was important; thus, the lower limbs were plased in sterile polyethylen tubes.
2.4. Tissue Processing and Histomorphometric Analysis
All the animals were weighted on 7th, 14th and 21st day after spinal cord transection. The rats were then sacrificed and ovaries were removed and their weight and volume were measured immediately and fixed in Boin’s and 10% formaldehyde solution. After fixation and processing of specimens, paraffin blocks were prepared and were serially sectioned with microtome (5 µm). The slides were stained by Hematoxylin and Eosin (H and E) and periodic acid schiff (PAS) methods (24).
For the purpose of an exact evaluation of ovarian quantitative changes, follicles were categorized, according to the criteria listed in the Nomina Histologica, as the following types: Perimordial Follicle (PF, monolayer of flattened follicular cells), Unilaminar Primary Follicle (UPF, monolayer of cuboidal granulosa cells), Multilaminar Primary Follicle (MPF, multilayer of cuboidal granulosa cells), Secondary Follicle (SF, oocyte surrounded by 2 or more granulosa cell layer, without an antrum) and graafian follicle or Tertiary Follicle (TF, oocyte surrounded by a stratified epithelium of granulosa cells with follicular antrum) (25). From each ovary, a transverse section at 10 fields was randomly selected at a ×400 magnification. Slides were studied by a light microscope equipped with a computerized image analysis system (Motic Images plus 2 Co., LTD). In each instance of the ovary, follicle and ovum diameter, thickness of zona pellocida, granulosa layer, and theca interna in all kinds of follicles were calculated.
2.5. Follicular Atresia
In this study, diagnoses of atretic follicles on the basis of the morphological criteria of the method were described by Greenwald and Roy (1994) in H and E stained serial sections of the ovary (26). The earliest sign of atresia was presence of 5% pyknotic granulosa cells in the largest cross section of the follicle.
2.6. Statistical Analysis
All data were expressed as means ± standard error of the mean (SEM). The quantitative data were analyzed using the SPSS software (Version 20). Data from experiments with more than 2 independent variables were analyzed using Analysis of Variance (ANOVA) followed by the Tukey-Kramer post-hoc tests. Significant differences were considered at P values of < 0.05.
3. Results
3.1. General Characteristic of Animals
Animals weight, mean volume, and weight of ovaries declined in spinal cord injury (SCI) rats in comparison with the sham and control groups. Decrease in animals weight was statistically significant (P < 0.05), (Table 1).
Body Weight, Ovarian Weight and Ovarian Volume of Control, Sham and Spinal Cord Injury Groups on the 7th, 14th and 21st Day After Spinal Cord Transection (N = 6)a
| Animal Groups | Body Weight, g | Ovarian Weight, mg | Ovarian Volume, cm3 |
|---|---|---|---|
| 7 days after surgery | |||
| Co | 223.8 ± 14.8 | 56.2 ± 3.3 | 0.059 ± 0.002 |
| Sh | 225.4 ± 12.4 | 55.9 ± 4.7 | 0.061 ± 0.005 |
| SCI | 208.5 ± 13.6b | 55.5 ± 2.7 | 0.058 ± 0.003 |
| 14 days after surgery | |||
| Co | 227.7 ± 8.4 | 60.7 ± 4.8 | 0.063 ± 0.005 |
| Sh | 219.2 ± 11.3 | 61.3 ± 3.9 | 0.062 ± 0.004 |
| SCI | 198.2 ± 10.9b | 59.3 ± 4.2 | 0.061 ± 0.003 |
| 21 days after surgery | |||
| Co | 238.4 ± 4.8 | 60.8 ± 5.6 | 0.064 ± 0.006 |
| Sh | 233.8 ± 5.8 | 60.1 ± 6.5 | 0.063 ± 0.006 |
| SCI | 201.8 ± 6.1b | 59.0 ± 5.2 | 0.061 ± 0.004 |
3.2. Histopathological Assessment
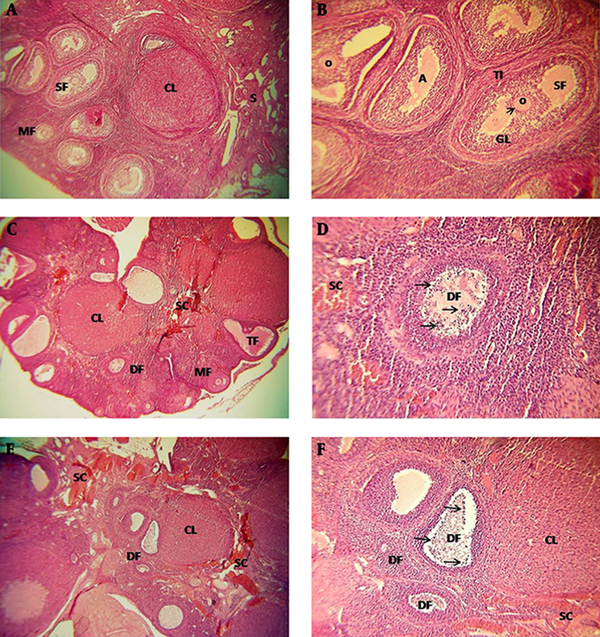
In the ovaries of the SCI group there were follicles at different stages of development and a number of corpus luteum consisting of irregular and heterogenous figures. Also, atretic follicles increased in SCI animals, specially on the 14th and 21st day (Figure 1A-F).
Almost all oocytes of SCI rats were pale stained and their nuclei were euchromatic, while the nucleoli were indistinguishable. In granulosa cells, the nuclous was spherical, centric, and euchromatic, and the cells had decreased cytoplasmic volume and eosinophillia (Figure 1C-F).
Degenerative changes including, pyknotic nucleus and vacuolated cytoplasm, were observed in some of the granulosa and theca interna cells. The amount of these cells increased specially on 14th and 21st day (Figure 1C-F). Cellular density, edema, fibrosis, and inflamation increased in ovarian stroma of SCI rats. Dilated and congested vessels were obvious specially in central stroma of the ovary and between theca interna and granulosa layer of early antral follicle and pre-ovulatory follicle (Figure 1C to F).
Light Micrographs of the Ovarian Structure in the Different Groups
3.3. Histomorphometric Assessment
The mean diameter of the follicle and ovum and the mean thickness of granulosa layer were significantly decreased in all types of follicles of the SCI group, specially on the 14th and 21st day after the operation compared with the control and sham groups (P < 0.05), (Figure 2A-C).
The mean thickness of theca interna in SF and TF and the mean thickness of zona pellocida in MPF, SF, and TF were significantly decreased in the SCI rats, specially on the 14th and 21st day after the operation compared with control and sham groups (P < 0.05), (Figure 2D and E).
Effects of Spinal Cord Injury on Ovarian Structure
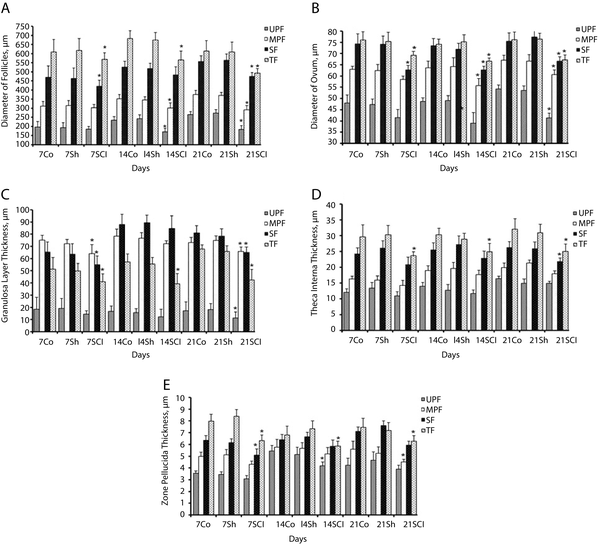
3.4. Follicular Atresia
Atreatic Unilaminar Primary Follicles (UPF) number were significantly more in SCI rats on 7th, 14th and 21st day than the control groups (Table 2). Atreatic Multilaminar Primary Follicles (MPF) number and Secondary Follicles (SF) were significantly more in SCI rats on 14th and 21st day than the control group. Also atreatic Tertiary Follicles (TF) number was significantly more in SCI rats on 21st day than the control groups (Table 2).
Mean Number of Atreatic Follicles in Control, Sham and Spinal Cord Injury Groups on the 7th, 14th, and 21st Day After Spinal Cord Transaction (N = 6)a
| Animal Groups | Mean Number of Atreatic Follicles / Ovary ± SEM | |||
|---|---|---|---|---|
| UPF | MPF | SF | TF | |
| 7 days after surgery | ||||
| Co | 13.30 ± 0.84 | 8.40 ± 0.53 | 7.20 ± 0.58 | 4.60 ± 1.12 |
| Sh | 14.10 ± 0.54 | 9.10 ± 0.45 | 7.80 ± 0.84 | 4.30 ± 0.94 |
| SCI | 19.40 ± 0.63b | 14.80 ± 0.78 | 11.20 ± 0.36 | 6.40 ± 0.82 |
| 14 days after surgery | ||||
| Co | 12.40 ± 0.78 | 9.20 ± 0.44 | 7.30 ± 0.82 | 5.40 ± 1.04 |
| Sh | 12.90 ± 066 | 9.80 ± 0.79 | 6.80 ± 0.58 | 4.70 ± 0.93 |
| SCI | 20.10 ± 0.76b | 15.60 ± 0.74b | 10.30 ± 0.47b | 7.30 ± 0.38 |
| 21 days after surgery | ||||
| Co | 15.60 ± 0.58 | 8.70 ± 0.48 | 6.50 ± 0.44 | 6.30 ± 0.57 |
| Sh | 14.80 ± 0.94 | 9.10 ± 0.54 | 7.10 ± 0.86 | 5.80 ± 0.82 |
| SCI | 23.20 ± 1.07b | 14.30 ± 0.72b | 12.10 ± 0.92b | 9.30 ± 0.38b |
4. Discussion
According to the results, animals weight, ovarian weight, and volume of the SCI rats decreased in comparison with Co and Sh rats, that corresponded with other studies. However, decrease in ovarian weight and volume during the acute phase after surgery was not significant.
Since gonadotropins have an important role in growth and development of ovarian follicles and corpus luteum, alteration in levels of gonadotropins after spinal cord transection, may lead to a decrease in ovarian weight and volume (16, 22, 24).
In the rats of the SCI group, severe changes in ovarian stroma, specially on the 21st day after the operation were observed, and the inflamation due to lack autonomic function and hyperprolactinemia after spinal cord transection may have caused these chenges. Following the inflammation and edema in ovary, then stromal fibroblasts are activated by Transforming growth factor beta (TGF-β), secreted by mast cells, thus collagen production increases and then fibrosis ensues in stroma. On the other hand, alteration in the amount of neurotransmitters and increase in prolactin and LH levels following the spinal cord transection may increase vascular permeability that in turn leads to edema and increase in stromal volume (14, 27).
This study proved that in all of the 3 subdivisions of the SCI group, the oocytes in all types of follicles were less dyeable, their nuclei were euchromatic, and the nucleoli were indistinguishable. Eosinophilia and cytoplasmic volume in granulosa layer and theca interna decreased and their nuclei became pale. Also, some of the cells with dense, small, and sometimes fusiform nuclei (possibly pyknotic cells) were visible.
Previous studies have shown that in addition to hypothalamus-pituitary-ovary axis, other factors including catecholamines and neurotransmitters via stimulation of β-adrenergic receptors in follicular cells and so increase of cAMP, interfere in regulation of follicular growth and development. Furthermore, ovarian nerves (superior ovarian nerve and ovarian plexus) in conjunction with neurotransmitters to cAMP connection and ovarian cholinergic neurons, consisting of Y neuropeptide, intervene in this process (8-11, 15, 28).
Following spinal cord transection, the regulatory role of nerves and neurotransmitters on growth and integrity of ovums, granulosa layer, and theca interna may be disrupted; thus, severe changes, with inhibitory affect on development and differentiation of ganulosa layer and theca interna, follow. Finally, these changes lead to pyknosis and increased ovarian hetrogenity.
This study proved that the main parameters of all kinds of follicles such as, the diameter of the follicle and ovum, thickness of granulosa layer, theca interna, and zona pellocida in the SCI group were exposed to severe changes. Increase in diameter of all types of follicles and thickness of granulosa layer, specially in graafian and primary follicles, was more severe in comparison with other parameters.
Since the CNS directly effects hormone regulation and ovarian development via spscific descending and ascending pathways and reasonable levels of gonadotropins are related to impulses from ovarian adrenergic nerves, it may be possible that spinal cord transection disrupts these connections and deranges synthesis and maturation of steroids hormones and also deranges regulation of ovarian hormones and as a result leads to histological and structural changes in all types of follicles (1, 8-10, 13, 29, 30).
4.1. Conclusion
The results suggest that spinal cord transection severely effects the ovaries and alters histology and structure of ovaries and increases their hetrogenity. These changes are due to disorder of gonadotropin regulation that is a result of autonomic function loss, alteration of catecholamines, and ovarian neurotransmitters level following spinal cord transection.
Acknowledgements
References
-
1.
Albright TH, Grabel Z, DePasse JM, Palumbo MA, Daniels AH. Sexual and Reproductive Function in Spinal Cord Injury and Spinal Surgery Patients. Orthop Rev (Pavia). 2015;7(3):5842. [PubMed ID: 26605025]. https://doi.org/10.4081/or.2015.5842.
-
2.
Sameni HR, Salsabili N, Hosseini A, Shariat SH, Rezazadeh M. Morphological and histological changes of rat prostate gland during acute phase of spinal cord transection [In Persian]. Kowsar Med J. 1997; 2:85-95.
-
3.
Boller F, Frank E. Sexual dysfunction in neurological disorders: diagnosis, management, and rehabilitation. New York: Raven Pr; 1982. p. 238-256.
-
4.
Nygaard I, Bartscht KD, Cole S. Sexuality and reproduction in spinal cord injured women. Obstet Gynecol Surv. 1990;45(11):727-32. [PubMed ID: 2234703].
-
5.
Anderson KD, Borisoff JF, Johnson RD, Stiens SA, Elliott SL. Long-term effects of spinal cord injury on sexual function in men: implications for neuroplasticity. Spinal Cord. 2007;45(5):338-48. [PubMed ID: 17016492]. https://doi.org/10.1038/sj.sc.3101978.
-
6.
Tederko P, Krasuski M, Kiwerski J. Impact of spinal injury with neurological consequences on sexual function: Sexual dysfunctions in women. Med Rehabil. 2007;11:23-6.
-
7.
DeForge D, Blackmer J, Garritty C, Yazdi F, Cronin V, Barrowman N, et al. Fertility following spinal cord injury: a systematic review. Spinal Cord. 2005;43(12):693-703. [PubMed ID: 15951744]. https://doi.org/10.1038/sj.sc.3101769.
-
8.
D'Albora H, Barcia JJ. Intrinsic neuronal cell bodies in the rat ovary. Neurosci Lett. 1996;205(1):65-7. [PubMed ID: 8867022]. https://doi.org/10.1016/0304-3940(96)12361-2.
-
9.
Woosely RM, Yoiung RR. Neurologic clinics disorders of the spinal cord. Saunders press; 1991. p. 275-323.
-
10.
D'Albora H, Lombide P, Ojeda SR. Intrinsic neurons in the rat ovary: an immunohistochemical study. Cell Tissue Res. 2000;300(1):47-56. [PubMed ID: 10805074]. https://doi.org/10.1007/s004410050046.
-
11.
Lawrence IJ, Burden HW. The origin of the extrinsic adrenergic innervation to the rat ovary. Anat Rec. 1980;196(1):51-9. [PubMed ID: 7416501]. https://doi.org/10.1002/ar.1091960106.
-
12.
Aguado LI, Ojeda SR. Prepubertal ovarian function is finely regulated by direct adrenergic influences. Role of noradrenergic innervation. Endocrinology. 1984;114(5):1845-53. [PubMed ID: 6325133]. https://doi.org/10.1210/endo-114-5-1845.
-
13.
Aguado LI, Ojeda SR. Ovarian adrenergic nerves play a role in maintaining preovulatory steroid secretion. Endocrinology. 1984;114(5):1944-6. [PubMed ID: 6538828]. https://doi.org/10.1210/endo-114-5-1944.
-
14.
Huang TS, Wang YH, Lai JS, Chang CC, Lien IN. The hypothalamus-pituitary-ovary and hypothalamus-pituitary-thyroid axes in spinal cord-injured women. Metabolism. 1996;45(6):718-22. [PubMed ID: 8637446]. https://doi.org/10.1016/S0026-0495(96)90137-7.
-
15.
Morales L, Chavez R, Ayala ME, Dominguez R. Effects of unilateral or bilateral superior ovarian nerve section in prepubertal rats on the ovulatory response to gonadotrophin administration. J Endocrinol. 1998;158(2):213-9. [PubMed ID: 9771465]. https://doi.org/10.1677/joe.0.1580213.
-
16.
Mayerhofer A, Frungieri MB, Bulling A, Fritz S. Sources and function of neuronal signalling molecules in the gonads. Medicina (B Aires). 1999;59(5 Pt 2):542-5. [PubMed ID: 10684155].
-
17.
Shunmugavel A, Khan M, Chou PC, Singh I. Spinal cord injury induced arrest in estrous cycle of rats is ameliorated by S-nitrosoglutathione: novel therapeutic agent to treat amenorrhea. J Sex Med. 2012;9(1):148-58. [PubMed ID: 22024253]. https://doi.org/10.1111/j.1743-6109.2011.02526.x.
-
18.
Osmanagaoglu MA, Usul H, Yulug E, Kesim M, Karahan SC. Hormonal and histological changes in the ovaries with high-doses of methylprednisolone administration for acute spinal cord injury: an experimental study. J Obstet Gynaecol. 2013;33(6):585-90. [PubMed ID: 23919856]. https://doi.org/10.3109/01443615.2013.789833.
-
19.
Sameni HR. The effects of spinal cord transection on prostate gland mast cells in rats [In Persian]. Koomesh. 2003;4(1):97-104.
-
20.
Linsenmeyer TA, Pogach LM, Ottenweller JE, Huang HF. Spermatogenesis and the pituitary-testicular hormone axis in rats during the acute phase of spinal cord injury. J Urol. 1994;152(4):1302-7.
-
21.
Linsenmeyer TA, Perkash I. Infertility in men with spinal cord injury. Arch Phys Med Rehabil. 1991;72(10):747-54. [PubMed ID: 1929799].
-
22.
Gerendai I, Toth IE, Boldogkoi Z, Medveczky I, Halasz B. CNS structures presumably involved in vagal control of ovarian function. J Auton Nerv Syst. 2000;80(1-2):40-5. [PubMed ID: 10742538]. https://doi.org/10.1016/S0165-1838(00)00071-0.
-
23.
Hubscher CH, Johnson RD. Effects of acute and chronic midthoracic spinal cord injury on neural circuits for male sexual function. II. Descending pathways. J Neurophysiol. 2000;83(5):2508-18. [PubMed ID: 10805652].
-
24.
Bancroft JD, Stevens A. Theory and practice of Histological techniques. Third ed. Churchill Livingstone; 1990. p. 21-81.
-
25.
Sreejalekshmi P, Raghavendra BS, Subramani TS, Murthy VC, Jamuna KV, Prasad RV, et al. Detection of follicular apoptosis in water buffalo (Bubalus bubalis) ovary by histology and nick end labelling technique. Reprod Domest Anim. 2011;46(1):59-65. [PubMed ID: 20070580]. https://doi.org/10.1111/j.1439-0531.2009.01569.x.
-
26.
Greenwald GS, Roy SK. Follicular development and its control. In: Knobil E, Neil JD, editors. The physiology of reproduction. 1. Ravan Press; 1994. p. 629-724.
-
27.
Yarkony GM, Novick AK, Roth EJ, Kirschner KL, Rayner S, Betts HB. Galactorrhea: a complication of spinal cord injury. Arch Phys Med Rehabil. 1992;73(9):878-80. [PubMed ID: 1514898].
-
28.
Wing TY, Christensen AK. Morphometric studies on rat seminiferous tubules. Am J Anat. 1982;165(1):13-25. [PubMed ID: 7137056]. https://doi.org/10.1002/aja.1001650103.
-
29.
Dominguez R. Differential ovulatory responses of the right and left ovaries of the adult rat to unilateral lesion and anaesthesia of the cervico-vaginal plexus. J Endocrinol. 1990;124(1):43-5. [PubMed ID: 2299277]. https://doi.org/10.1677/joe.0.1240043.
-
30.
Gordon JR, Galli SJ. Promotion of mouse fibroblast collagen gene expression by mast cells stimulated via the Fc epsilon RI. Role for mast cell-derived transforming growth factor beta and tumor necrosis factor alpha. J Exp Med. 1994;180(6):2027-37. [PubMed ID: 7964480]. https://doi.org/10.1084/jem.180.6.2027.