Abstract
Objectives:
The objective of this study was to evaluate if ozone is capable of improving pain, function and quality of life, and to decrease serum uric acid in human knee osteoarthritis (OA) patients.Methods:
Overall, 42 patients, 31 females (73.81%) and 11 males (26.19%) were enrolled in a prospective quasi experimental before-and-after study. Mean age of the sample was 66.9 ± 8.83 years. Treatment consisted of four sessions (one per week) of an intra articular infiltration of a medical mixture of oxygen-ozone (95% to 5%), 20 mL volume at a 20 µ/mL concentration, on the most symptomatic painful knee. Before and after the intervention, the researchers measured outcomes including serum uric acid and pain, function, and quality of life by the visual analogue scale (VAS) and by Western Ontario and Mac Master index for OA (WOMAC). The OA patients were graded by Kellgren-Lawrence radiological scale as 2° to 4° grades.Results:
Serum uric acid decreased from 5.19 ± 1.22 mg/dL to 5.03 ± 1.22 (P = 0.0439). The WOMAC pain subscale score decreased from 14.26 ± 2.61 to 5.95 ± 2.97 points (P = 0.0001), WOMAC stiffness subscale diminished from 2.72 ± 1.63 to 1.04 ± 1.04 points (P = 0.0001), and WOMAC function subscale improved from 41.78 ± 10.17 to 24.61 ± 9.86 points (P = 0.0001).Conclusions:
Intra articular ozone is capable of decreasing pain and stiffness and improving function and quality of life, as well as decreasing serum uric acid in knee OA patients.Keywords
1. Background
Osteoarthritis (OA) is the most common type of arthritis and it is a chronic, painful, and inflammatory disease that produces functional impairment in Western societies and has affected nearly 27 million Americans and 4 million Spanish patients, to date some (1-3).
The economic impact is such that OA is the cause of 50% of disabilities in Spain, and the direct cost in this country is 4 738 million Euros per year, representing 0.5% of the gross domestic product (3). Furthermore, OA is a progressive and degenerative age-related joint disease that culminates in loss of articular cartilage, joint space narrowing, and remodeling of subchondral bone (4). Osteoarthritis has no cure and the current management includes non-pharmacological and pharmacological interventions and frequently involves costly joint replacement procedures, not exempt from risk or side effects (3, 5).
Osteoarthritis pathogenesis is multifactorial. Risk factors include gender, race, overweightness, load bearing, obesity, traumatisms, and recently, low-grade chronic inflammation (3, 6, 7). Inflammation has increasingly been recognized as a driver of OA disease pathology, thus implicating the synovial environment, including the role of inflammatory cytokines and infiltrated immune cells, driving the degeneration of cartilage tissue (7-9).
The challenge for the future in the management of OA is to discover early tools for the diagnosis and prognosis of the disease, and to discover effective treatments. Biomarkers constitute those early tools (10). Since OA is closely related to inflammation, inflammation biomarkers, such as C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), pro-inflammatory cytokines (IL-6, IL-1β), and very recently uric acid has been used to determine such relationship (11-14). Several epidemiological observations have reported the association of uric acid with OA. Roddy et al. suggested that gout and OA share a common pathogenic link (15). Ma et al. stated that the deposition of monosodium uric acid crystals secondary to hyperuricemia, promote direct cartilage degradation (14). Martinon and Denoble, in different studies, stated that monosodium uric acid crystals activate macrophage innate immune responses via NALP3 (Natch Domain, Leucin-rich repeat, and pyrin domain containing protein 3), activating caspase-1 and releasing IL-1β and IL-18, cytokines related to cartilage degradation. According to this study, gout and OA would share the same inflammatory pathway (14, 16, 17).
Biomarkers of inflammation (especially CRP and IL-6) and biomarkers of cartilage degradation (including matrix metalloproteinases), have been previously related to pain and progression in OA (18-21). Besides, non-steroidal anti-inflammatory drugs (NSAIDs) and dietary bioactive compounds (curcumin, ginger, green tea polyphenols, and strawberries) have been shown to be effective in the management of pain symptoms and in reducing inflammatory biomarkers of OA (22). Unfortunately, most of such studies for the management of knee OA are limited in number and short in duration; moreover, they mainly evaluate pain and function but no inflammation biomarkers.
Decades of experience have demonstrated that ozone is capable of modulating inflammation (23). Fernandez-Cuadros et al. stated that intra articular ozone is useful in the management of knee OA, improving pain, function, and quality of life (24). In a two-year follow-up period, the same study group stated that ozone is capable of slowing and even reverting knee OA, based on radiographic evaluation (25, 26). Very recently, the current researchers reported the efficacy of ozone in improving knee OA symptoms and decreasing surrogate biomarkers of inflammation, namely CRP and ESR in human patients with knee OA (26). However, to the best of the author’s knowledge, there is no report on the efficacy of ozone in decreasing uric acid, a new biomarker of inflammation in knee OA patients.
The objective of the current study was to evaluate if ozone is capable of improving pain, function, and quality of life (QoL), and to decrease serum uric acid in human knee OA patients.
2. Methods
A total of 42 patients were enrolled in a prospective quasi-experimental before-and-after study; 31 females (73.81%) and 11 males (26.19%). Mean age of the sample was 66.9 ± 8.83 years (Table 1). The study was performed at the rehabilitation department of Santa Cristina University Hospital and run from January, 2017 to March, 2018, and it was approved by the Ethical Committee of the Santa Cristina University Hospital.
Principal Demographical, Clinical and Biochemical Variables of the Patients Studied at Baseline (N = 42)
| Variables | Value |
|---|---|
| Age, mean ± SD, y | 66.90 ± 8.83 |
| Female, frequency (%) | 31 (73.81%) |
| Male, frequency (%) | 11 (26.19%) |
| Ratio, female:male ratio | 3:1 |
| OA KL 2°, No. (%) | 26 (61.9%) |
| OA KL 3°, No. (%) | 10 (23.8%) |
| OA KL 4°, No. (%) | 6 (14.3%) |
| Uric acid, mg/dL , mean ± SD | 5.19 ± 1.22 |
| Uric acid, mmol/mL , mean ± SD | 308 ± 72 |
| VAS (0 - 10) | 7.02 ± 1.20 |
| WOMAC pain (0 - 20) | 14.26 ± 2.61 |
| WOMAC stiffness (0 - 8) | 2.72 ± 1.63 |
| WOMAC function (0 - 68) | 41.78 ± 10.17 |
Patients older than 18 years of age, with clinical signs of OA (based on the criteria of the American College of rheumatology) (27) and on radiological signs, graded from 2° to 4° (based on Kellgren and Lawrence (KL) grading system), were included in the study. Patients with allergy to ozone (23-26) or those, who did not perform biochemical, clinical or radiological follow-up evaluation were excluded from the study.
Before treatment, informed consent was obtained and signed by all patients. The severity of pain was measured by the visual analog scale (VAS), function and quality of life were measured by the WOMAC scale (Western Ontario and Mac Master Index for Osteoarthritis), and serum uric acid was evaluated by biochemical analysis.
The VAS is a Likert type pain scale used to evaluate pain in a graded score from 0 to 10, 0 meaning no pain, and 10 the greatest pain experienced by the patient (28). Furthermore, WOMAC is an Index that evaluates pain, function, and stiffness in knee OA patients and it is useful for evaluating the specific quality of life in these patients. This scale evaluates three items, namely pain (form 0 to 20), stiffness (from 0 to 8), and function (from 0 to 68), where a greater value indicates greater severity (3, 29).
Depending on the radiographic appearance of knee joints, patients are categorized in four categories, according to the KL grading system. Grade 0: normal, grade 1: doubtful narrowing of the joint space and possible osteophyte lipping, grade 2: osteophytes and joint space narrowing, grade 3: moderate multiple osteophytes, definite narrowing of joint space, some sclerosis and possible deformity of the bone contour, grade 4: large osteophytes with marked narrowing of the joint space, severe sclerosis and definite deformity of the bone contour (30).
Serum uric acid determination was performed by the use of the 3P39-41 Uric Acid Reagent Kit ® (from Abbot, USA). The serum was collected by standard venipuncture techniques. The coefficient of variation of this determination was 3.6% (31).
The treatment protocol consisted of four sessions (one session/week) of an intra articular infiltration of a medical mixture of oxygen-ozone (95% - 5%), 20 mL volume at a 20-µg/mL concentration. Patients were infiltrated on the most symptomatic knee. The technique has been previously described by the current study group (23-26).
2.1. Statistical Analysis
After the fourth infiltration was performed, VAS and WOMAC scales were applied, serum uric acid was analyzed and adverse effects were registered. To perform the statistical evaluation, the statistical package for social studies (SPSS) 20.0® was used. Mean and standard deviation (SD) were used to describe quantitative variables at baseline; while, for qualitative variables, frequencies, and percentages were employed. To evaluate change, before and after the intervention, T-student Test was applied. The level of significance was 95% (P = 0.05).
3. Results
A total of 42 patients were analyzed in the current study. Female patients accounted for 73.81% (n = 31) and male patients represented 26.19% (n = 11). Female to male ratio was 3:1 (Table 1). Mild and moderate knee OA was more frequent than severe OA. That is, OA KL 2° (n = 26, 61.9%) was more frequent than OA KL 3° (n = 10, 23.8%) and OA KL 4° (n = 6, 14.3%) (Table 1).
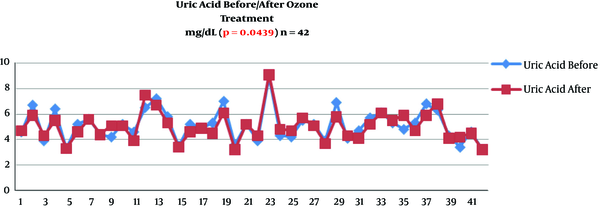
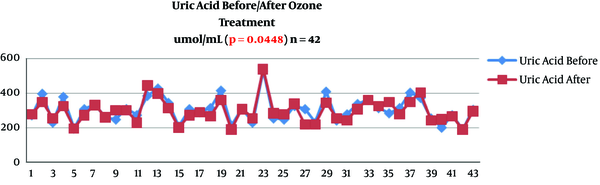
Serum uric acid decreased its value from 5.19 ± 1.22 mg/dL to 5.03 ± 1.22 (P = 0.0439) (Table 2 and Figure 1). Serum uric acid decreased from 308 ± 75 to 297 ± 69 mmol/mL (P = 0.0448) (Table 2 and Figure 2).
| Variables | Before | After | P Valueb |
|---|---|---|---|
| Uric acid, mg/dL | 5.19 ± 1.22 | 5.03 ± 1.22 | 0.0439 |
| Uric acid, mmol/mL | 308 ± 72 | 297 ± 69 | 0.0448 |
| VAS (0 - 10) | 7.02 ± 1.20 | 2.97 ± 1.48 | 0.0001 |
| WOMAC pain (0 - 20) | 14.26 ± 2.61 | 5.95 ± 2.97 | 0.0001 |
| WOMAC stiffness (0 - 8) | 2.72 ± 1.63 | 1.04 ± 1.04 | 0.0001 |
| WOMAC rigidity (0 - 68) | 41.78 ± 10.17 | 24.61 ± 9.86 | 0.0001 |
Change of serum uric acid after ozone therapy (mg/dL) in knee Osteoarthritis patients (n = 42)
Change of serum uric acid after ozone therapy (mmol/mL) in knee osteoarthritis patients (n = 42)
Ozone decreased pain measured by VAS from 7.02 ± 1.2 to 2.97 ± 1.48 points (P = 0.0001). Ozone improved all items of the WOMAC scale after ozone treatment. The WOMAC pain subscale decreased from 14.26 ± 2.61 to 5.95 ± 2.97 points (P = 0.0001), WOMAC stiffness subscale diminished from 2.72 ± 1.63 to 1.04 ± 1.04 points (P = 0.0001), and WOMAC function subscale improved from 41.78 ± 10.17 to 24.61 ± 9.86 points (P = 0.0001) (Table 2).
With respect to different knee OA grades, serum uric acid levels were not related to knee OA severity. Serum uric acid levels were similar in mild, moderate, and severe knee OA patients. On the contrary, pain, function, and QoL worsened as knee OA progressed based on knee OA KL grades. However, after ozone treatment, both serum uric acid levels and clinical symptoms (namely pain, function, and QoL) improved after the intervention, in all knee OA grades (Table 3).
Change in Variables Depending on Knee Osteoarthritis Severity (Kellgren Lawrence Grades 2°, 3° and 4°) Before-and-After Ozone Therapy (N = 42)a
| Variable | OA KL 2° (N = 26) | OA KL 3° (N = 10) | OA KL 4° (N = 6) | |||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| Uric acid mg/dL | 5.33 ± 1.08 | 5.25 ± 0.96 | 4.58 ± 1.03 | 4.42 ± 0.79 | 5.2 ± 1.98 | 5.11 ± 2.1 |
| Uric acid mmol/mL | 316 ± 64 | 308 ± 60 | 271 ± 61 | 262 ± 46 | 309 ± 118 | 304 ± 125 |
| VAS | 6.76 ± 1.3 | 2.8 ± 1.29 | 7.3 ± 0.94 | 3.5 ± 1.71 | 7.6 ± 1.03 | 2.8 ± 2.04 |
| WOMAC pain | 13.76 ± 2.83 | 5.61 ± 2.59 | 14.6 ± 1.89 | 7 ± 3.43 | 15.8 ± 2.56 | 5.6 ± 4.08 |
| WOMAC stiffness | 2.34 ±1.74 | 0.76 ± 0.95 | 3.5 ± 1.5 | 1.7 ± 0.94 | 3.3 ± 0.81 | 1.16 ± 1.32 |
| WOMCA function | 38.96 ± 9.64 | 21.69 ± 9.17 | 45.8 ± 7.68 | 29.5 ± 8.2 | 47.3 ± 13.6 | 29.16 ± 12.5 |
4. Discussion
To the best of the author’s knowledge, this is the first study that states that Ozone decreases serum uric acid, as a new OA biomarker, and the decrease comes in line with a clinical improvement on symptoms severity, such as pain, stiffness, function, and QoL.
Uric acid is the end product of purine metabolism in humans and it is generated by the action of xanthine oxidase enzyme. For years, uric acid was considered as a metabolically inert substance; however, growing evidence states that uric acid has multiple actions on cellular metabolism (32). 1, Uric acid acts as an endogenous antioxidant and powerful scavenger of single oxygen peroxyl (ROS) and hydroxyl radical (OH) (33); 2, inside the cell, uric acid exerts pro-oxidative effects and behaves as a pro inflammatory factor (32). Thus, uric acid can act as an antioxidant and pro-oxidant factor. When acting as an antioxidant, uric acid chelates metals and scavenges oxygen radicals (34). As a pro-oxidant, uric acid oxidizes lipids, reduces nitric oxide availability in endothelial cells, (35) and increases reactive oxygen species (36). In summary, under normal physiologic conditions, uric acid acts as an antioxidant; yet, under ischemic conditions, becomes a pro-oxidant, producing systemic inflammation, common in systemic diseases, such as metabolic syndrome, hypertension, stroke, atherosclerosis, and recently OA (32).
This comes in line with the growing evidence that uric acid is related to markers of systemic inflammation. Several population-based studies in healthy males and females showed that serum uric acid is positively associated with CRP (37). A study of 957 elderly Italian individuals showed that serum uric acid is positively associated with CRP, and also tumor necrosis factor (TNF)-α, and interleukin (IL)-6 (38). In another study that included 608 Caucasians from Switzerland, serum uric acid was found to be positively associated with CRP, TNF-α, and IL-6 (in both males and females) (39). Another study Giovine et al. mentioned that uric acid may stimulate the production of TNF-α in synovial cells (32).
As stated before, uric acid is related to systemic diseases and systemic biomarkers; yet there is also plenty of evidence that relates uric acid to OA (14). Acheson et al. stated that uric acid was associated with hand OA in females. Anderson et al. suggested that uric acid was associated with increased knee OA in females. Sun et al. observed that highest uric acid level was associated with generalized OA in previous hip OA patients. Ding et al. claimed that higher uric acid level was associated with osteophytes in females. Bagge et al. communicated that uric acid was associated with knee OA in females. Schouten et al. indicated that higher uric acid levels were associated with loss of joint space width. Krasnokutsky et al. suggested that uric acid was associated with joint space narrowing. Roddy et al. observed that gout attacks were associated with the presence of OA. Howard et al. stated that gout was associated with knee OA, and knee OA was more severe in gout patients. Tang et al. reported that gout was associated with total knee replacement in females (14).
All previous evidence suggests that uric acid and OA might share some common pathogenesis pathways. There are two possible mechanisms that would explain the pathological link between uric acid and OA. In the first mechanism, gout may promote cartilage degradation due to the direct effects of monosodium urate crystals. Crystal’s deposits were strongly associated with cartilage degradation. By a second mechanism, acid uric crystals might activate the macrophage innate immune response via NALP3 inflammasome, releasing IL-1β and IL-18, after activation of caspase-1 (14).
In a very interesting study, Denoble stated that synovial fluid is a dialysate of serum fluid; and in that study, it was observed that serum uric acid concentrations were associated with synovial uric acid concentrations. Moreover, this study reported that the soluble form of uric acid in synovial fluid was strongly associated with synovial fluid IL-1β and IL-18. This study also suggested that synovial uric acid, IL-1β, and IL-18 were associated with knee OA graded by radiography and bone scintigraphy (17). Denoble et al. also hypothesized that uric acid, either diffused into the joint from systemic circulation or released from dying chondrocytes, forms micro-particles that trigger the innate immunity and NALP3 inflammation pathway (14, 17). This is in accordance with Wangkaew, who stated that synovial fluid is a dialysate of blood plasma because synovial membrane permits uric acid and other small particles to pass freely through the double barrier of endothelium and interstitium into the synovial fluid (39). In fact, Wangkaew compared the uric acid concentration in serum and synovial fluid from several arthritides (rheumatoid arthritis, septic arthritis, OA, ankylosing spondylitis and calcium pyrophosphate dehydrate deposition disease), and the values were very similar; the ratio of serum/synovial fluid uric acid concentration was nearly 1.0 (39).
As seen before, uric acid is related to biomarkers of inflammation; namely CRP, IL-6, and TNF-α, but also to synovial fluid IL-1β and IL-18. Since ozone is capable of modulating inflammation, and the current study group had recently reported that ozone reduces biomarkers of inflammation, the authors hypothesized that ozone could be capable of decreasing uric acid, an objective that has been demonstrated in the current study and reported for the first time in the literature.
In the current study, it was observed that patients with knee OA showed a level of uric acid of 5.19 mg/dL or 308 mmol/mL. Denoble reported in her series a 6.0-mg/dL of Uric Acid level (17), while Wangkaew referred a 6.2-mg/mL level (39). Krasnokutsky reported a serum uric acid level of 6.3 mg/dL in 88 OA patients (40). Srivastava published on patients with knee OA a 5.2-mg/dL of uric acid level, which is very similar to the current study (41). In posttraumatic knee OA, Panina et al. reported serum uric acid levels of 5.78 mg/dL or 344 mmol/mL (42). Ding et al. stated that female patients with knee OA have lower levels of uric acid (275 mmol/mL) if compared with male patients (362 mmol/mL) (43).
The previously reported values are noteworthy, because patients were non-gout; and although uric acid levels are lower than 6.8 mg/dL (which is the crystallization threshold), this level is good enough to contribute to cartilage degradation, via activation of inflammasomes (17). This would explain the symptoms referred by patients at baseline in the current study, measured by VAS and WOMAC scales.
The strengths of the current study were the use of a standardized clinical method for measuring serum uric acid levels, the standardized acquisition of radiographs to classify knee OA, and the use of validated clinical scales, such as VAS and WOMAC. The sample was mostly community-based and probably representative of patients with knee OA in the general population.
A limitation of the current study was the lack of control group. This was mainly due to the limited number of cases (n = 42). As patients accepted the ozone protocol, and they failed previous conservative treatment, it was not ethical to deny an ozone intervention. A before and after intervention is a methodology used to solve such ethical situation and to solve the absence of the control group. Thus, a before-and-after evaluation was performed on the same treatment group. In such a case, the change observed after the intervention is expected to be a direct effect of the ozone treatment protocol. However, the methodological limitation of the study and the small sample size do not affect the observations of this study.
4.1. Conclusion
Intra articular ozone is capable of decreasing pain and stiffness and improving function and quality of life, while decreasing serum uric acid in knee OA patients. The biochemical and clinical effectiveness of ozone has been observed in mild, moderate, and severe knee OA grades.
Acknowledgements
References
-
1.
Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012;64(4):465-74. [PubMed ID: 22563589].
-
2.
Meneses SR, Goode AP, Nelson AE, Lin J, Jordan JM, Allen KD, et al. Clinical algorithms to aid osteoarthritis guideline dissemination. Osteoarthritis Cartilage. 2016;24(9):1487-99. [PubMed ID: 27095418]. https://doi.org/10.1016/j.joca.2016.04.004.
-
3.
Fernandez-Cuadros ME, Perez-Moro OS, Albaladejo-Florin MJ; Miron-Canelo. Ozone fundamentals and effectiveness on knee pain: Chondromalacia and knee Osteoarthritis. Germany: Lambert Academic Publishing; 2016.
-
4.
Tonge DP, Pearson MJ, Jones SW. The hallmarks of osteoarthritis and the potential to develop personalised disease-modifying pharmacological therapeutics. Osteoarthritis Cartilage. 2014;22(5):609-21. [PubMed ID: 24632293]. https://doi.org/10.1016/j.joca.2014.03.004.
-
5.
McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014;22(3):363-88. [PubMed ID: 24462672]. https://doi.org/10.1016/j.joca.2014.01.003.
-
6.
Fernandez-Cuadros ME. Analisis de la calidad de vida en pacientes con prótesis total de rodilla. Spain: Universidad de Salamanca; 2013.
-
7.
Pearson MJ, Herndler-Brandstetter D, Tariq MA, Nicholson TA, Philp AM, Smith HL, et al. IL-6 secretion in osteoarthritis patients is mediated by chondrocyte-synovial fibroblast cross-talk and is enhanced by obesity. Sci Rep. 2017;7(1):3451. [PubMed ID: 28615667]. [PubMed Central ID: PMC5471184]. https://doi.org/10.1038/s41598-017-03759-w.
-
8.
Deligne C, Casulli S, Pigenet A, Bougault C, Campillo-Gimenez L, Nourissat G, et al. Differential expression of interleukin-17 and interleukin-22 in inflamed and non-inflamed synovium from osteoarthritis patients. Osteoarthritis Cartilage. 2015;23(11):1843-52. [PubMed ID: 26521730]. https://doi.org/10.1016/j.joca.2014.12.007.
-
9.
Moradi B, Rosshirt N, Tripel E, Kirsch J, Barie A, Zeifang F, et al. Unicompartmental and bicompartmental knee osteoarthritis show different patterns of mononuclear cell infiltration and cytokine release in the affected joints. Clin Exp Immunol. 2015;180(1):143-54. [PubMed ID: 25393692]. [PubMed Central ID: PMC4367102]. https://doi.org/10.1111/cei.12486.
-
10.
Parera IM, Gharbi M, Serrano HM, Barbero MH, Milano JV, Henrotin Y. Effect of chondroitin sulfate on soluble biomarkers of osteoarthritis: How to analyze and interpret the results from an open-label trial in unilateral knee osteoarthritis patients. Basic & Clinical Pharmacology & Toxicology. 2015;117.
-
11.
Saxne T, Lindell M, Mansson B, Petersson IF, Heinegard D. Inflammation is a feature of the disease process in early knee joint osteoarthritis. Rheumatology (Oxford). 2003;42(7):903-4. [PubMed ID: 12826709]. https://doi.org/10.1093/rheumatology/keg214.
-
12.
Sowers M, Jannausch M, Stein E, Jamadar D, Hochberg M, Lachance L. C-reactive protein as a biomarker of emergent osteoarthritis. Osteoarthritis Cartilage. 2002;10(8):595-601. [PubMed ID: 12479380].
-
13.
Malathi R. Raised serum IL 6 and CRP in radiographic knee osteoarthritis in Eastern India. J Med Sci clin Res. 2017;5(5):21687-92. https://doi.org/10.18535/jmscr/v5i5.73.
-
14.
Ma CA, Leung YY. Exploring the Link between Uric Acid and Osteoarthritis. Front Med (Lausanne). 2017;4:225. [PubMed ID: 29326934]. [PubMed Central ID: PMC5733531]. https://doi.org/10.3389/fmed.2017.00225.
-
15.
Roddy E, Doherty M. Gout and osteoarthritis: a pathogenetic link? Joint Bone Spine. 2012;79(5):425-7. [PubMed ID: 22867976]. https://doi.org/10.1016/j.jbspin.2012.03.013.
-
16.
Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440(7081):237-41. [PubMed ID: 16407889]. https://doi.org/10.1038/nature04516.
-
17.
Denoble AE, Huffman KM, Stabler TV, Kelly SJ, Hershfield MS, McDaniel GE, et al. Uric acid is a danger signal of increasing risk for osteoarthritis through inflammasome activation. Proc Natl Acad Sci U S A. 2011;108(5):2088-93. [PubMed ID: 21245324]. [PubMed Central ID: PMC3033282]. https://doi.org/10.1073/pnas.1012743108.
-
18.
Perruccio AV, Chandran V, Power JD, Kapoor M, Mahomed NN, Gandhi R. Systemic inflammation and painful joint burden in osteoarthritis: a matter of sex? Osteoarthritis Cartilage. 2017;25(1):53-9. [PubMed ID: 27546883]. https://doi.org/10.1016/j.joca.2016.08.001.
-
19.
Larsson S, Englund M, Struglics A, Lohmander LS. Interleukin-6 and tumor necrosis factor alpha in synovial fluid are associated with progression of radiographic knee osteoarthritis in subjects with previous meniscectomy. Osteoarthritis Cartilage. 2015;23(11):1906-14. [PubMed ID: 26521736]. https://doi.org/10.1016/j.joca.2015.05.035.
-
20.
Ling SM, Patel DD, Garnero P, Zhan M, Vaduganathan M, Muller D, et al. Serum protein signatures detect early radiographic osteoarthritis. Osteoarthritis Cartilage. 2009;17(1):43-8. [PubMed ID: 18571442]. [PubMed Central ID: PMC2667202]. https://doi.org/10.1016/j.joca.2008.05.004.
-
21.
Pelletier JP, Raynauld JP, Caron J, Mineau F, Abram F, Dorais M, et al. Decrease in serum level of matrix metalloproteinases is predictive of the disease-modifying effect of osteoarthritis drugs assessed by quantitative MRI in patients with knee osteoarthritis. Ann Rheum Dis. 2010;69(12):2095-101. [PubMed ID: 20570834]. https://doi.org/10.1136/ard.2009.122002.
-
22.
Schell J, Scofield RH, Barrett JR, Kurien BT, Betts N, Lyons TJ, et al. Strawberries Improve Pain and Inflammation in Obese Adults with Radiographic Evidence of Knee Osteoarthritis. Nutrients. 2017;9(9). [PubMed ID: 28846633]. [PubMed Central ID: PMC5622709]. https://doi.org/10.3390/nu9090949.
-
23.
Fernández-Cuadros ME, Pérez-Moro OS, Mirón-Canelo JA. Could ozone be used as a feasible future treatment in osteoarthritis of the knee? Diversity and Equality in Health and Care. 2016;13(3).
-
24.
Fernández Cuadros ME, Pérez Moro OS, Albaladejo Florin MJ, Mirón Canelo JA. Ozone improves pain, function and quality of life in patients with knee osteoarthritis: A prospective quasi-experimental before-after study. Middle East J Rehabil Health. 2016;4(1). https://doi.org/10.17795/mejrh-41821.
-
25.
Fernandez-Cuadros ME, Susana Perez-Moro O, Jesus Albaladejo-Florin M. Knee osteoarthritis: Condroprotector action and symptomatic effect of ozone on pain, function, quality of life, minimal joint space and knee arthroplasty delay. Middle East J Rehabil Health. 2006;4(1). https://doi.org/10.17795/mejrh-43200.
-
26.
Fernández-Cuadros ME, Pérez-Moro OS, Albaladejo-Florín MJ. Ozone decreases biomarkers of inflammation (C-reactive protein and erythrocyte sedimentation rate) and improves pain, function and quality of life in knee osteoarthritis patients. A before-and-after study. Middle East J Rehabil Health. 2018.
-
27.
Peat G, Thomas E, Duncan R, Wood L, Hay E, Croft P. Clinical classification criteria for knee osteoarthritis: performance in the general population and primary care. Ann Rheum Dis. 2006;65(10):1363-7. [PubMed ID: 16627539]. [PubMed Central ID: PMC1798313]. https://doi.org/10.1136/ard.2006.051482.
-
28.
Burckhardt CS, Jones KD. Adult measures of pain: the McGill pain questionnaire (MPQ), rheumatoid arthritis pain scale (RAPS), Short‐Form McGill pain questionnaire (SF‐MPQ), verbal descriptive scale (VDS), visual analog scale (VAS), and West Haven‐Yale multidisciplinary pain inventory (WHYMPI). Arthritis Care Res. 2003;49(S5). https://doi.org/10.1002/art.11440.
-
29.
Woolacott NF, Corbett MS, Rice SJ. The use and reporting of WOMAC in the assessment of the benefit of physical therapies for the pain of osteoarthritis of the knee: findings from a systematic review of clinical trials. Rheumatology (Oxford). 2012;51(8):1440-6. [PubMed ID: 22467082]. https://doi.org/10.1093/rheumatology/kes043.
-
30.
Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494-502. [PubMed ID: 13498604]. [PubMed Central ID: PMC1006995]. https://doi.org/10.1136/ard.16.4.485.
-
31.
Tholen DW, Kallner A, Kennedy JW, Krouwer JS, Meier K. Evaluation of precision performance of quantitative measurement methods; approved guideline-second edition. 2004.
-
32.
Billiet L, Doaty S, Katz JD, Velasquez MT. Review of hyperuricemia as new marker for metabolic syndrome. ISRN Rheumatol. 2014;2014:852954. [PubMed ID: 24693449]. [PubMed Central ID: PMC3945178]. https://doi.org/10.1155/2014/852954.
-
33.
Sautin YY, Nakagawa T, Zharikov S, Johnson RJ. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am J Physiol Cell Physiol. 2007;293(2):C584-96. [PubMed ID: 17428837]. https://doi.org/10.1152/ajpcell.00600.2006.
-
34.
So A, Thorens B. Uric acid transport and disease. J Clin Invest. 2010;120(6):1791-9. [PubMed ID: 20516647]. [PubMed Central ID: PMC2877959]. https://doi.org/10.1172/JCI42344.
-
35.
Bagnati M, Perugini C, Cau C, Bordone R, Albano E, Bellomo G. When and why a water-soluble antioxidant becomes pro-oxidant during copper-induced low-density lipoprotein oxidation: a study using uric acid. Biochem J. 1999;340 ( Pt 1):143-52. [PubMed ID: 10229669]. [PubMed Central ID: PMC1220232]. https://doi.org/10.1042/bj3400143.
-
36.
Khosla UM, Zharikov S, Finch JL, Nakagawa T, Roncal C, Mu W, et al. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005;67(5):1739-42. [PubMed ID: 15840020]. https://doi.org/10.1111/j.1523-1755.2005.00273.x.
-
37.
Bonora E, Targher G, Zenere MB, Saggiani F, Cacciatori V, Tosi F, et al. Relationship of uric acid concentration to cardiovascular risk factors in young men. Role of obesity and central fat distribution. The Verona Young Men Atherosclerosis Risk Factors Study. Int J Obes Relat Metab Disord. 1996;20(11):975-80. [PubMed ID: 8923153].
-
38.
Lyngdoh T, Marques-Vidal P, Paccaud F, Preisig M, Waeber G, Bochud M, et al. Elevated serum uric acid is associated with high circulating inflammatory cytokines in the population-based Colaus study. PLoS One. 2011;6(5). e19901. [PubMed ID: 21625475]. [PubMed Central ID: PMC3098830]. https://doi.org/10.1371/journal.pone.0019901.
-
39.
Wangkaew S, Kasitanon N, Hongsongkiat S, Tanasombat C, Sukittawut W, Louthrenoo W. A comparative study of serum and synovial fluid levels of uric acid between patients with gout and other arthritides. J Med Assoc Thai. 2014;97(7):679-85. [PubMed ID: 25265764].
-
40.
Krasnokutsky S, Oshinsky C, Attur M, Ma S, Zhou H, Zheng F, et al. Serum Urate Levels Predict Joint Space Narrowing in Non-Gout Patients With Medial Knee Osteoarthritis. Arthritis Rheumatol. 2017;69(6):1213-20. [PubMed ID: 28217895]. [PubMed Central ID: PMC5449226]. https://doi.org/10.1002/art.40069.
-
41.
Srivastava RN, Sanghi D, Mishra A, Sharma A, Raj S, Natu S. Serum uric acid as a predisposing factor of clinico- radiological severity of osteoarthritis knee. Osteoarthr Cartil. 2013;21. S251. https://doi.org/10.1016/j.joca.2013.02.517.
-
42.
Panina SB, Krolevets IV, Milyutina NP, Sagakyants AB, Kornienko IV, Ananyan AA, et al. Circulating levels of proinflammatory mediators as potential biomarkers of post-traumatic knee osteoarthritis development. J Orthop Traumatol. 2017;18(4):349-57. [PubMed ID: 29058227]. [PubMed Central ID: PMC5685991]. https://doi.org/10.1007/s10195-017-0473-8.
-
43.
Ding X, Zeng C, Wei J, Li H, Yang T, Zhang Y, et al. The associations of serum uric acid level and hyperuricemia with knee osteoarthritis. Rheumatol Int. 2016;36(4):567-73. [PubMed ID: 26743214]. https://doi.org/10.1007/s00296-015-3418-7.