Abstract
Keywords
Meigs Syndrome Ascites Hydrothorax Ovarian Cancer Pleural Effusion
1. Introduction
Classically Meigs syndrome is described as a pleural effusion with or without ascites associated with an ovarian fibroma that resolves when the tumor is removed (1). This observation only occurs in 1% of cases, yet in fibromas larger than 10 cm, this association is described in up to 10% to 15% of cases (1). Therefore, Meigs syndrome is a rare nosological entity that appears in adult females (2). Although the association of ascites and pleural effusion (hydrothorax) with benign ovarian tumors was already known since the 19th century, it was not until 1937 when Meigs and Cass reported seven cases and seven months later, Rhodes and Terrel described them for the first time as Meigs’ syndrome (3).
The classic clinical-pathological picture of Meigs syndrome is characterized by the association between ovarian fibroma, pleural effusion, and ascites (2). The first cases corresponded to ovarian fibromas, yet after a review of the cases in 1954, Meigs added to fibromas, thecomas, granulosa cell tumors or the Brenner tumor as “benign ovarian tumors” that may cause this syndrome (3). The treatment of the Meigs Syndrome involves the resection of the tumor, after which the spontaneous resolution of the pleural effusion (hydrothorax) and peritoneal effusion (ascites) occurs (2, 3).
Meigs’ Pseudo-syndrome is defined as the association with tumors of histological characteristics different from the classic description, either benign or malignant (2, 3).
The objective of this case report was to describe the infrequent appearance of sudden massive pleural effusion in a patient after a cytoreductive laparotomy of an ovarian adenocarcinoma, which in the context of the patient (malignant ovarian tumor and ascites) confirms the presence of a Pseudo-Meigs Syndrome. In addition, the probable pathophysiological causes of pleural and peritoneal effusion are analyzed and in this particular case, conservative treatment (oral medication and respiratory physiotherapy) was considered.
2. Case Presentation
From the Rehabilitation Department, the researchers went on to evaluate a 57-year-old female with ovarian cancer (mucinous adenocarcinoma) and ascites, who underwent surgery two days before hand in order to try to perform cytoreduction of a giant tumor that occupied the right hemiabdomen. The reason for the consultation was to request respiratory rehabilitation since the patient presented dyspnea and non-productive nocturnal cough that did not improve with the use of aerosols (after 24 hours of evolution). The patient, currently in her intermediate-recovery unit persisted with a non-productive cough.
As a background, the patient was a smoker (20 cigarettes/day) and a drinker for 20 years. A year ago she complained of asthenia, weight loss, and increased abdominal perimeter. Two months ago, a 5-L evacuation paracentesis was performed. The tumor markers were: CEA 10.82 ng/mL (elevated), AFP 3.14 ng/mL (Normal), CA 199 9645.8 U/mL (very high), CA 125 37.4 U/mL (slightly elevated), and CA 153 14.2 U/m (Normal). The cytology was negative for tumor cells. The tomography revealed a complex cystic lesion in the right iliac fossa, probably of ovarian origin, measuring 15 cm and with a large amount of accompanying ascites (Figure 1). One month ago, a diagnostic laparoscopy confirmed a very complex multicystic tumor of 15 cm in the right iliac fossa and the biopsy confirmed adenocarcinoma with mucinous differentiation, probably of ovarian origin and the cytology of peritoneal fluid was inflammatory, although the culture revealed Staphylococcus simulans (infection of peritoneal fluid). Once the possibility of adjuvant treatment (chemotherapy or radiotherapy) due to peritoneal infection was ruled out, it was decided to perform cytoreductive laparotomy, following antibiotic therapy (Linezolid + Clindamycin + Gentamicin). Two days ago, medial laparotomy was performed with release of adhesions and removal of giant ovarian tumor (29 × 25 × 22 cm), which was completely removed yet from the oncological point of view (oncologic surgery was planned for omentectomy, pelvic and para-aortic lymphadenectomy, appendectomy, hysterectomy and resection of all visible disease), the result was sub-optimal, thus the surgery was terminated.
In the planning of the cuts in the tomography scan (left image) the increase in the abdominal perimeter of the patient was observed and the abdominal examination confirmed semiologic signs of ascites (displaceable dullness on palpation). Tomography scan (right image) revealed a complex cystic lesion in the right iliac fossa, probably of ovarian origin, measuring 15 cm and with a large amount of accompanying ascites.

Upon examination, the patient was awake and oriented, with a 96% O2 saturation. Tachypnea was observed (respiratory rate of 24 per minute). At the pulmonary examination, auscultatory silence was observed, and the percussion was not tympanic (dull) in the right hemithorax. The absence of vesicular murmur and transmitted vocal vibrations was confirmed with the stethoscope. The auscultation of the left hemithorax revealed no abnormal aggregate noises. Upon examination of the abdomen, the globular abdomen was seen on inspection with displaceable dullness on palpation, compatible with ascites (Figure 1). In addition, there were venous dilatations in the abdominal wall.
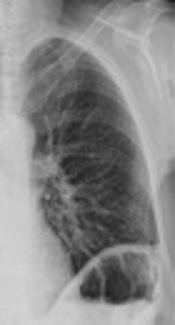
There was no type of alteration in the previous control of chest radiograph two days ago, or in the previous tomography (Figure 2). The chest radiograph revealed a massive opacification of the right hemithorax with tracheal displacement to the left side. A linear image corresponding with left pleural effusion was also observed. The jugular catheter was in the right atrial entrance (Figure 2). The radiologist’s report confirmed a right pleural effusion and complete atelectasis of the right lung.
The radiograph prior to laparotomy (left image) shows clean lung fields discarding some radiological sign of pleural effusion. The radiograph 2 days after the cytoreductive laparotomy (right image) confirms massive pleural effusion, with total atelectasis of the right lung and deviation of the trachea towards the right hemithorax. A line of pleural effusion is seen in the left lung field. The jugular catheter is seen at the entrance to the right atrium.

After anamnesis, clinical examination and evaluation of the diagnostic tests, the diagnostic assessment was (1) Complete lung atelectasis secondary to (2) Probable massive pleural effusion (hydrothorax), and (3) Ascites, in the context of (4) Pseudo- Meigs syndrome of recent appearance. The therapeutic plan, given the patient’s tolerance to dyspnea and the time of evolution (convalescent from a recent laparotomy), was to perform inspiratory exercises according to tolerance to maintain the ventilator mechanics of the left hemithorax. Depending on the evolution of the case and/or clinical worsening, evacuation thoracocentesis was suggested. After seven days of evolution, the decrease of the effusion was partial, yet given the pluri pathology, the severity and the condition of the patient, evacuating thoracocentesis was ruled out, and conservative medical treatment and continued respiratory physiotherapy was chosen (Figure 3).
After conservative treatment (oral medication, fluid restriction, and respiratory physiotherapy) the control radiographs at 3 (top left), 5 (top right) and 7 (bottom) days revealed the partial but progressive improvement of pulmonary atelectasis. Given the severity of the patient, evacuator thoracocentesis was ruled out.

3. Discussion
As far as the authors are concerned, this is the first article describing the sudden presence of a Pseudo-Meigs Syndrome in a patient after a cytoreductive laparotomy of a mucinous cystadenocarcinoma of the ovary. The importance of the case is the rapid establishment of the effusion and the infrequent presentation since there are very few cases of mucinous cystadenocarcinomas reported in the literature, such as those published by Long et al., Maiga et al., Gil et al., and Losa et al. (4).
Ovarian cancer is the sixth most frequent malignancy in females and responsible for the highest number of deaths per year from cancer of the female reproductive system. Mortality is high because diagnosis is usually established in advanced stages (5). It is a disease that mainly influences postmenopausal women, with a median age at diagnosis of 60 years (5), in the current case, 57 years.
Ovarian cancer represents the highest mortality of all gynecological cancers (2). Before 1937, patients with ovarian tumor and the presence of pleural effusion (hydrothorax) were considered inoperable, considering patients in cancer stage IV (2). However, in 1937 Meigs and Cass described a series of seven patients with ovarian fibromas, ascites, and hydrothorax, in whom signs and symptoms resolved after the removal of their tumors (2).
The knowledge of Meigs syndrome is of considerable clinical importance because an ovarian tumor with ascites and pleural fluid suggests malignancy and may result in peritoneal and pleural metastases (2). Subsequently to its definition, Meigs (in 1954) described the association of these signs and symptoms with thecomas, granulosa cell tumors, and Brenner tumors (6). When these signs appear to be associated with a non-ovarian pelvic mass or a tumor different from those previously described (struma-like teratomas, cysts, tubal papilloma, and malignant tumors such as papillary cystadenoma, Krukenberg tumor, carcinoma or fibrosarcoma), the disease is called pseudo-Meigs syndrome (2, 5, 7).
Meigs Syndrome is a rare entity (about 4% of benign ovarian tumors), which occurs in women of middle age (3). Its differentiation with a Pseudo-Meigs Syndrome is relevant because in case of a Meigs Syndrome, surgical resection of the pelvic mass would produce a spontaneous resolution of the pleural and peritoneal effusion (2, 5).
Although Meigs’ syndrome has been known for more than 80 years, its pathophysiology is still unclear and several theories have been proposed to explain the origin of ascites and pleural effusion (hydrothorax), although none has been fully investigated in depth (4). The prevalence of ascites is 10% to 15% in ovarian fibromas, which only accounts for 2% to 5% of the pieces resected by the ovarian tumor, so its frequency is very low (5). With respect to hydrothorax, it has been seen that it may be unilateral or bilateral, yet it occurs more frequently on the right side (62% right, 24% bilateral, and 11% left) (4, 5).
The cause of the formation of ascites and pleural effusion is unknown, yet López-Sánchez et al. have suggested that peritoneal irritation or inflammation caused by the tumor could contribute to the formation of ascites, while the presence of communications between the pleura and the peritoneum would be responsible for the formation of the pleural effusion and the hydrothorax (2, 5). Sánchez-Torres has proposed different mechanisms, such as (a) irritation and inflammation of the peritoneum by a hard ovarian mass, (b) pressure in the veins and lymphatic vessels of the peritoneal cavity, and (c) hormonal stimulation and release of mediators from the tumor that increase capillary permeability; although this data is still being investigated (5).
In favor of the inflammatory theory, there are several studies that argue that various cytokines and growth factors play a role in the pathogenesis of the syndrome, since increasing capillary permeability would contribute to the formation of ascites and pleural effusion (4). In this regard, Abramov observed that Interleukins (IL) IL-1β, IL-6, IL-8, Vascular Endothelial Growth Factor (VEGF), Fibroblast Growth Factor (FGF) and Tumor Necrosis Factor (TNF-α) were elevated in the plasma, in the ascetic and pleural fluid of a 62-year-old patient with Meigs’ syndrome before surgery; and all these cytokines (except TNF-α) decreased to normal values after surgery, coinciding with the disappearance of ascites and hydrothorax (8).
In favor of the theory of diffusion of ascitic fluid into the pleural space by the diaphragm making use of the lymphatic vessels and the cellular interstitium, a electrophoresis study demonstrated the passage of a dye (India ink) from the abdomen to the thorax, and not the other way around (4). (4).
In favor of the theory of defects in the diaphragmatic peritoneum membrane, in a scintigraphy study with macroaggregated albumin labeled with Tc99m at the intra-abdominal level, it was observed that after 20 minutes, part of the labeled radiotracer passed to the right thoracic cavity, which demonstrates the existence of peritoneum-pleural communication (9). Lopez et al. argue that the origin of hydrothorax (massive pleural effusion) is due to the passage of ascitic fluid from the abdominal cavity to the pleura through congenital or acquired aponeurotic defects in its tendinous portion favored by negative intrathoracic pressure (10). The right hemidiaphragm has more tendinous tissue and less muscle component than the left, which would explain the high frequency of right unilateral hemithorax (10). When the accumulation of fluid in the pleural space exceeds the absorption capacity of the pleura, the hydrothorax (pleural effusion greater than 500cc) is produced (10).
The current researchers believe that this mechanism (peritoneal pleural defect) has been responsible for the sudden presence of massive pleural effusion (hydrothorax) in the patient in the current case, and that after cytoreductive laparotomy, this defect would have been permeabilized after extraction of the tumor mass, explaining in this way the rapid and massive appearance of unilateral hydrothorax. In addition, the rapid appearance of dyspnea and dry cough in this patient would be explained by the volume of pleural fluid that in our patient was massive and produced total pulmonary collapse, two days after surgery.
The majority of authors maintain that pleural effusion and ascites disappear spontaneously after surgery without any specific treatment (2, 4). The resolution of pleural effusion and ascites usually occurs several days after surgery, with a range of three to fourteen days after surgery (according to Meigs), although it may take up to about six months (4, 6). In the current case, there was a partial resolution after seven days of evolution.
Regarding the treatment of pleural effusion, although the diaphragmatic defect can spontaneously close, thus resolving the effusion, it is normal to persist; therefore initially conservative treatment is recommended (9), based on oral medication (diuretics), hydro electrolytic restriction (salt), and respiratory physiotherapy (inspiratory and expiratory exercises) to adequately maintain the ventilator mechanics of the affected hemithorax, and so the researchers proceeded in their clinical case. In case of non-response to drug therapy or severe dyspnea, evacuator thoracentesis is the rescue means to be considered (9). However, thoracentesis can be associated with electrolyte alterations, hypovolemia, and the development of infection (9). In the current case, given the severity of the patient, and tolerance to dyspnea, evacuation thoracocentesis was ruled out.
3.1. Conclusions
Meigs syndrome is associated with the clinical triad of ascites, pleural effusion/hydrothorax, and ovarian tumor (fibroma, thecoma, granulosa cell tumor, and Brenner tumor); while the presence of any tumor other than those described is classified as Pseudo-Meigs Syndrome. In the current case, the sudden presentation of massive pleural effusion with massive pulmonary atelectasis after cytoreductive laparotomy in the context of a patient with mucinous adenocarcinoma of the ovary and ascites, is explained by Pseudo-Meigs Syndrome. The pathophysiology of ascites and pleural effusion is currently controversial (inflammatory theories, dissemination and diaphragmatic defect); however, in the current case, the diaphragmatic defect at the peritoneum-pleural level would explain the suddenness of its presentation. Conservative management and respiratory physiotherapy have partially improved this complication. Given the severity of the patient, more aggressive measures (evacuator thoracocentesis) were ruled out.
Acknowledgements
References
-
1.
López Vilaro L, Muñoz R, Murillo R, Gimeno J, Baró T, García P, et al. Síndrome de Meigs y peritonitis esclerosante: Una asociación poco frecuente. Progresos de Obstetricia y Ginecología. 2010;53(11):480-4. https://doi.org/10.1016/j.pog.2010.07.001.
-
2.
López Sánchez P, García Escobar JL, Rius Jordà J, Laforga J, Torregrosa P. Síndrome de Meigs: Presentación de dos casos. Progresos de Obstetricia y Ginecología. 2002;45(9):403-7. https://doi.org/10.1016/s0304-5013(02)75812-3.
-
3.
Nemeth AJ, Patel SK. Meigs syndrome revisited. J Thorac Imaging. 2003;18(2):100-3. [PubMed ID: 12700485].
-
4.
Losa EM, Villar M, Pascual A, Gómez T, Gónzalez de Merlo G. Síndrome de Meigs y seudo-Meigs. Clínica e Investigación en Ginecología y Obstetricia. 2006;33(1):25-34. https://doi.org/10.1016/s0210-573x(06)74078-3.
-
5.
Sánchez-Torres DA, Díaz-Murillo R, Kazlauskas S, de Santiago J, Zapardiel I. Síndrome de Meigs por fibroma ovárico bilateral parecido al cáncer de ovario. Ginecologia y Obstetricia de Mexico. 2016;84(2).
-
6.
Meigs JV. Fibroma of the ovary with ascites and hydrothorax-Meigs' syndrome. Am J Obstetr Gynecol. 1954;67(5):962-87. https://doi.org/10.1016/0002-9378(54)90258-6.
-
7.
Kazanov L, Ander DS, Enriquez E, Jaggi FM. Pseudo-Meigs' syndrome. Am J Emerg Med. 1998;16(4):404-5. https://doi.org/10.1016/s0735-6757(98)90141-3.
-
8.
Abramov Y, Anteby SO, Fasouliotis SJ, Barak V. Markedly elevated levels of vascular endothelial growth factor, fibroblast growth factor, and interleukin 6 in Meigs syndrome. Am J Obstet Gynecol. 2001;184(3):354-5. [PubMed ID: 11228486]. https://doi.org/10.1067/mob.2001.110028.
-
9.
Fernandez Font JM, de Miguel Diez J, Opio Maestro V. [Hepatic hydrothorax]. An Med Interna. 2005;22(5):253-4. Spanish. [PubMed ID: 16089018].
-
10.
López P, Mainardi V, Kierszenbaum M, Alonso J. Hidrotórax hepático: Cuando la evolución es la evidencia. Archivos de Medicina Interna. 2012;34(1):5.