Abstract
Background:
Although studies have shown that vitamin D deficiency in pregnancy is associated with gestational diabetes mellitus (GDM), the clinical evidence of the preventive role of high-dose vitamin D supplementation in pregnancy is in doubt.Objectives:
The study aimed to evaluate the effect of vitamin D supplementation on the GDM occurrence.Methods:
In a randomized clinical trial conducted in Semnan, Iran, in 2014, we recruited 175 eligible pregnant women at 8 - 12 weeks with vitamin D deficiency and normal fasting blood sugar (FBS) and randomly divided them into intervention (n = 87) and control groups (n = 88). From the 14 to 16 weeks, both groups received the 400 IU/daily doses of vitamin D. The intervention group received an additional 50,000 IU every two weeks for six weeks. In the 24 - 26 weeks, we measured FBS, serum vitamin D level, and oral glucose tolerance (75 g).Results:
The baseline levels of serum vitamin D (mean ± SD) were 15.38 ± 7.69 and 14.00 ± 8.81 (P = 0.728) in the intervention and control groups, respectively. There was no difference between the two groups in severe vitamin D deficiency (27.6% vs. 30.7%, P = 0.652). After the intervention, the serum vitamin D level was significantly different between the two groups (16.63 ± 6.77 vs. 66.96 ± 21.13, P < 0.001). However, it did not reach the toxic serum level in any of the participants. The level of vitamin D in 85 (97.7%) and 0 (0.0%) participants reached the normal range; there were 0 (0.0%) and 16 (18.8%) cases of severe deficiency in the groups. Twelve women (13.6%) in control and eight (9.2%) in intervention groups developed GDM (P = 0.356).Conclusions:
A high dose of vitamin D supplementation at 14th - 16th weeks of pregnancy could improve vitamin D deficiency effectively and safely. Despite the decreased incidence of GDM, there was no significant evidence of its preventive role.Keywords
Vitamin D Deficiency Gestational Diabetes Mellitus Glucose Tolerance Test
1. Background
Gestational diabetes mellitus (GDM) is known as glucose intolerance starting or being diagnosed during pregnancy (1). It occurs in 4.9 to 6.9% of pregnancies (2). Diabetes and pregnancy are deeply correlated, affecting the mother’s health (3).
Vitamin D has significant effects on the health of the mother and fetus (4). It regulates mineral homeostasis of calcium and phosphorus (5), adjusts the transfer of calcium through the placenta, stimulates immunological and anti-microbial activities (6), and affects the fetus’s brain development, childhood diseases, and psychological health during adulthood (7, 8). Vitamin D deficiency may lead to different pregnancy complications such as insulin resistance and GDM (9-11), hypertension and preeclampsia (12, 13), bacterial vaginosis, and cesarean section (14). Fetal complications include the loss of bone density and growth delay (15), preterm birth, low birth weight, neonatal respiratory infections, higher rates of human immunodeficiency virus transmission (16), asthma, eczema (17), osteoporosis, type I diabetes (18), autism (19), multiple sclerosis (20), and some autoimmune diseases in adults (4-11, 13, 21-26).
Many studies have shown the association between vitamin D deficiency and impaired glucose metabolism (27). Vitamin D can have a major role in insulin secretion and function (28) and GDM occurrence (29). There is a vivid reverse relationship between serum vitamin D levels and GDM (9, 12-20, 27, 28, 30, 31) so that women with vitamin D deficiency in early pregnancy have 3.7 times more risk of GDM than women with normal levels (11). Despite a large number of studies on vitamin D deficiency in pregnancy, there are a few studies on pregnant women to evaluate the effect of vitamin D supplementation on GDM. Until now, the normal range of vitamin D in pregnancy is unknown and its safe, effective dose for mothers and fetuses is under investigation (22, 32-36).
2. Objectives
The study aimed to evaluate the effect of high-dose vitamin D supplementation on compensation for vitamin D deficiency in pregnancy and the occurrence of GDM.
3. Methods
This randomized clinical trial was conducted at Amiralmomenin Hospital in Semnan, Iran, in 2014 (RCT registration code: IRCT2015022714275M2 and Research Ethics certificate approval ID: IR.SEMUMS.REC.1398.107). The inclusion criteria included 16-35-years-old pregnant women, gestational age (GA) of 8 to 12 weeks based on the last menstrual period or sonography, singleton pregnancy, body mass index (BMI) < 30 in the first prenatal visit, fasting blood sugar (FBS) ≤ 92, and vitamin D serum levels of < 32 ng/mL. In this study, vitamin D levels of ≤ 10 ng/mL were considered as severely deficient, 10 - 32 ng/mL as inadequate, and 32 - 100 as normal. The values of above 100 were conservatively considered as toxic values (37). The exclusion criteria included known metabolic and malabsorption diseases, GDM history, persistent glucosuria, parathyroid diseases, untreated thyroid or other endocrinologic complications, known hepatic and renal problems, history of polyhydramnios, macrosomia (> 4 kg), malformation, stillbirth, smoking, and the use of anti-epileptics, corticosteroids, and other drugs that might influence calcium and vitamin D metabolism. We measured FBS with an in-vitro enzymatic assay kit (GOD-PAPkit by Parsazmun Company, Tehran, Iran) and serum levels of 25-hydroxyvitamin D by ELISA. By setting the vitamin D deficiency correction at 20% as the primary outcome (21) and the power and confidence level at 80% and 95%, respectively, and considering the percentage of lost-to-follow-up of 12%, the sample size was determined as 180 women.
After obtaining written informed consent, we randomized participants into control and intervention groups of 90 using permuted block random allocation. During the 14th to 16th weeks of pregnancy, participants received a multi-prenatal pill of 400 IU of vitamin D. A 50,000 IU vitamin D pearl was also given to the intervention group once every two weeks (a total of six pearls). If the intervention group members forgot to take any dosages of pearls, they had to take it immediately and if they missed two consecutive doses, they would be excluded. The symptoms of vitamin D toxicity were described to the patients.
We measured serum vitamin D levels during 24th-26th gestational weeks and performed the Oral Glucose Tolerance test (OGTT) with 75 grams of glucose. FBS ≤ 92, or blood sugar ≤ 180 one hour after taking 75 grams of glucose, or blood sugar ≤ 153 two hours after taking 75 grams of glucose were the criteria of GDM, only one of which was sufficient for diagnosis. In the presence of GDM or abnormal vitamin D levels, we referred patients to an endocrinologist for further treatment. The safety of the intervention was defined based on the serum level of vitamin D and not exceeding the normal range.
The frequency (%) was reported for categorical variables and to evaluate their relationships with groups, the chi-square test was applied. For quantitative variables, median and inter-quartile range (IQR) were reported while the Mann-Whitney U test was used to compare the groups before and after the intervention. For further clarification, the mean and standard deviation (SD) were reported for vitamin D and FBS levels. We used SPSS v.16 for all analyses and the P value below 0.05 was considered significant.
4. Results
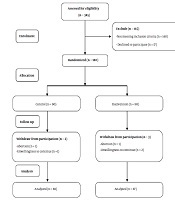
Among 345 women, 180 with eligibility criteria were randomly allocated to equal control and intervention groups. Two cases of control and three cases of intervention were excluded from the study. Finally, the data of 175 participants (87 in the intervention group and 88 in the control group) were analyzed (Figure 1).
Fellow diagram of participants in the intervention and control groups from enrollment to analysis

The median (IQR) of age was 28 (6) and 27 (5.7) in the control and intervention groups, respectively. There was no significant difference in age (P = 0.206), BMI (P = 0.232), and obstetrical characteristics (P > 0.05) between the two groups (Table 1).
| Characteristics | Intervention (N = 87) | Control (N = 88) | P Valueb |
|---|---|---|---|
| Age | 28 (6) | 27 (5.8) | 0.206 |
| Body mass Index | 26 (5) | 24 (4.9) | 0.232 |
| Parity | 1 (1) | 1 (1) | 0.820 |
| Gravidity | 0 (1) | 0 (1) | 0.982 |
| Living child | 0 (1) | 0 (1) | 0.979 |
| Stillbirth | 0 (0) | 0 (0) | 0.994 |
| Abortion | 0 (0) | 0 (0) | 0.507 |
| Fasting blood sugar, mg/mL | 83 (11) | 82 (10) | 0.516 |
| Serum vitamin D | 14 (15.1) | 17 (12.2) | 0.728 |
The mean and SD in the two groups were 82.5 ± 6.7 mg/dL and 81.3 ± 8.15 mg/dL for FBS and 14 ± 8.8 ng/mL and 15.3 ± 7.6 ng/mL for vitamin D. There was no significant difference in FBS (P = 0.516) and vitamin D (P = 0.728) before the intervention. Initially, 27 cases of intervention and 24 cases of control had severe vitamin D deficiency (P = 0.652) (Table 2).
| Level, ng/mL | Before | P Value | After | P Valueb | ||
|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | |||
| Normal (> 32) | 0 (0.0) | 0 (0.0) | 0.652 | 85 (97.7) | 0 (0.0) | < 0.001 |
| Insufficient (10 - 32) | 61 (69.3) | 63 (72.4) | 2 (2.3) | 69 (81.2) | ||
| Sever deficient (< 10) | 27 (30.7) | 24 (27.6) | 0 (0.0) | 16 (18.8) | ||
After the intervention, the mean and SD of vitamin D level in the intervention and control groups were 66.9 ± 21 ng/mL and 16.6 ± 6.77 ng/mL, respectively. There was a significant increase in the level of vitamin D after the intervention (P < 0.001). There were no significant differences between the two groups at the 24th - 26th gestational weeks in FBS (P = 0.162), one-hour OGTT (P = 0.996), and two-hour OGTT (P = 0.154) (Table 3).
| Characteristics | Intervention (N = 87) | Control (N = 88) | P Valueb |
|---|---|---|---|
| Serum vitamin D | 66 (35.8) | 17 (9.1) | < 0.001 |
| Change in serum vitamin D | 47 (29) | 0.3 (8.9) | < 0.001 |
| FBS | 81 (10) | 78 (10) | 0.162 |
| BS (1-h) | 125 (35) | 125 (39) | 0.996 |
| BS (2-h) | 109 (33) | 104 (26.5) | 0.154 |
The level of vitamin D reached the normal range in 97.7% of the intervention group and there was no severe deficiency in the remnants. It did not reach the toxic serum level in any of the participants. Twelve (13.6%) in the control group and eight (9.2%) in the intervention group developed GDM. Although the incidence of GDM was lower in the intervention group, there was no statistically significant difference (P = 0.356).
5. Discussion
Recently due to the high prevalence of vitamin D deficiency, lots of studies were carried out on the issue (24, 26, 38). However, studies on pregnant women are quite limited. The normal range of vitamin D in pregnant women is still in the process of research (22, 34, 39). Our study showed that the use of 50,000 IU vitamin D once every two weeks up to six doses led to a range of 32 - 100 ng/mL in 98% of vitamin D deficient pregnant women. In women who used 400 IU vitamin D daily, the vitamin D level remained in the range of deficiency. Since none of the participants reached the toxic levels, the intervention was considered to be safe. The number of cases with severe deficiency decreased from 27.6% to 18.8%. However, it was not statistically significant. The administration of 50,000 IU vitamin D once every two weeks led the average 47 ng/mL rise in vitamin D serum levels while 400 IU daily led to only 0.30 ng/mL rise.
Yap et al. (39) showed that the serum levels of 90% of the daily users of 5,000 IU and 66% of the daily users of 400 IU of vitamin D reached > 20 ng/mL. Nevertheless, in their study, 10% of the cases remained deficient. This is while in our study, only 2% of the intervention group was diagnosed with deficiency after treatment and none of them was severely deficient. The lack of cooperation in daily supplementation versus weekly supplementation may be the cause of this result. Asemi et al. (40, 41) showed that supplementation with 400 IU daily after 25 weeks led to the rise of serum levels but half of the cases remained below 20 ng/mL. In another study, supplementation with two doses of 50,000 units every three weeks in diabetic pregnant women, without considering the primary level, led to a significant rise of serum vitamin D levels (41).
In Soheilikhah et al. (42) study, just 50,000 IU every two weeks led to the normal serum levels in severe vitamin D deficient pregnant women while 200 IU daily did not compensate for the deficiency. Because of the fat solubility of vitamin D, daily or weekly doses have the same effect (43). Due to the compliance, we propose 50,000 IU of vitamin D once every two weeks for vitamin D deficient pregnant women. The recommended 400 IU daily dose of vitamin D in pregnancy (44) can only be used for prevention and cannot compensate for the deficiency.
Although the compensation for vitamin D deficiency reduced the rate of GDM from 13.6% to 9.2%, this difference was not statistically significant (P = 0.356). Several studies showed lower vitamin D levels in diabetic pregnant women (9, 11, 45). Zang et al. (11) concluded that women with vitamin D deficiency in early pregnancy were 3.7 times more likely to have GDM. Poel et al. (30) meta-analysis demonstrated that although its serum levels had a wide spectrum (6.6 - 39.7 ng/mL), pregnant women who had lower serum levels of vitamin D had a high likelihood of GDM.
Several studies have recently been done on pregnant women to investigate the effect of different doses of vitamin D supplementation on glycemic indices (40-42, 44, 46). In some studies, the use of vitamin D in GDM with/without vitamin D normal serum levels improved the function of pancreatic beta cells and decreased serum insulin, insulin resistance, and FBS. Soheilykhah et al. (42) studied 120 pregnant women below 12 weeks with severe vitamin D deficiency in three groups: A, 200 IU daily; B, 50,000 IU monthly; and C, 50,000 IU once every two weeks. They showed improvement in insulin resistance in all participants but it was significant in group C compared to group A. They showed no improvement in FBS. Hosseinzadeh-Shamsi-Anar et al. (46) showed that a single 300,000 IU dose of intramuscular vitamin D led to the rise of vitamin D serum levels but it did not have any effect on HbA1c. In our study, although the FBS values of both groups decreased, it was not statistically significant (P = 0.162).
Some studies (40, 44, 46) showed that vitamin D supplementation could normalize glycemic indices only in cases with diabetes risk factors or the ones who already suffered from it. Our study and Yap et al. (39) study showed that vitamin D supplementation might not influence the GDM incidence. The contradictory results of our study and the studies mentioned above may be due to the elimination of the cases with diabetes risk factors.
The main limitation of the present study was inadequate power due to the low sample size (calculated power = 0.16). Considering our results, with a statistical power of 0.80, at least two groups of 700 cases are required.
5.1. Conclusions
High-dose vitamin D supplementation in vitamin D deficient pregnant women (50,000 IU every two weeks from 14 - 16 weeks) could efficiently restore vitamin D deficiency. However, there is not enough evidence to suggest it as a preventive measure for GDM.
References
-
1.
Cunningham F, Leveno K, Bloom S, Dashe J, Hoffman B, Casey B, et al. Williams obstetrics. 25th ed. New York: McGraw Hill; 2018. p. 1098-9.
-
2.
Rajput R, Yadav Y, Nanda S, Rajput M. Prevalence of gestational diabetes mellitus & associated risk factors at a tertiary care hospital in Haryana. Indian J Med Res. 2013;137(4):728-33. [PubMed ID: 23703340]. [PubMed Central ID: PMC3724253].
-
3.
Goldman M, Kitzmiller JL, Abrams B, Cowan RM, Laros RJ. Obstetric complications with GDM. Effects of maternal weight. Diabetes. 1991;40 Suppl 2:79-82. [PubMed ID: 1748271]. https://doi.org/10.2337/diab.40.2.s79.
-
4.
Barrett H, McElduff A. Vitamin D and pregnancy: An old problem revisited. Best Pract Res Clin Endocrinol Metab. 2010;24(4):527-39. [PubMed ID: 20832734]. https://doi.org/10.1016/j.beem.2010.05.010.
-
5.
Mahon P, Harvey N, Crozier S, Inskip H, Robinson S, Arden N, et al. Low maternal vitamin D status and fetal bone development: cohort study. J Bone Miner Res. 2010;25(1):14-9. [PubMed ID: 19580464]. [PubMed Central ID: PMC4768344]. https://doi.org/10.1359/jbmr.090701.
-
6.
Liu N, Kaplan AT, Low J, Nguyen L, Liu GY, Equils O, et al. Vitamin D induces innate antibacterial responses in human trophoblasts via an intracrine pathway. Biol Reprod. 2009;80(3):398-406. [PubMed ID: 19005165]. [PubMed Central ID: PMC2704027]. https://doi.org/10.1095/biolreprod.108.073577.
-
7.
McGrath JJ, Burne TH, Feron F, Mackay-Sim A, Eyles DW. Developmental vitamin D deficiency and risk of schizophrenia: A 10-year update. Schizophr Bull. 2010;36(6):1073-8. [PubMed ID: 20833696]. [PubMed Central ID: PMC2963051]. https://doi.org/10.1093/schbul/sbq101.
-
8.
O'Loan J, Eyles DW, Kesby J, Ko P, McGrath JJ, Burne TH. Vitamin D deficiency during various stages of pregnancy in the rat; its impact on development and behaviour in adult offspring. Psychoneuroendocrinology. 2007;32(3):227-34. [PubMed ID: 17276604]. https://doi.org/10.1016/j.psyneuen.2006.12.006.
-
9.
Aghajafari F, Nagulesapillai T, Ronksley PE, Tough SC, O'Beirne M, Rabi DM. Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: Systematic review and meta-analysis of observational studies. BMJ. 2013;346:f1169. [PubMed ID: 23533188]. https://doi.org/10.1136/bmj.f1169.
-
10.
Ramos-Lopez E, Kahles H, Weber S, Kukic A, Penna-Martinez M, Badenhoop K, et al. Gestational diabetes mellitus and vitamin D deficiency: Genetic contribution of CYP27B1 and CYP2R1 polymorphisms. Diabetes Obes Metab. 2008;10(8):683-5. [PubMed ID: 18476984]. https://doi.org/10.1111/j.1463-1326.2008.00879.x.
-
11.
Zhang C, Qiu C, Hu FB, David RM, van Dam RM, Bralley A, et al. Maternal plasma 25-hydroxyvitamin D concentrations and the risk for gestational diabetes mellitus. PLoS One. 2008;3(11). e3753. [PubMed ID: 19015731]. [PubMed Central ID: PMC2582131]. https://doi.org/10.1371/journal.pone.0003753.
-
12.
Bodnar LM, Catov JM, Simhan HN, Holick MF, Powers RW, Roberts JM. Maternal vitamin D deficiency increases the risk of preeclampsia. J Clin Endocrinol Metab. 2007;92(9):3517-22. [PubMed ID: 17535985]. [PubMed Central ID: PMC4288954]. https://doi.org/10.1210/jc.2007-0718.
-
13.
Robinson CJ, Alanis MC, Wagner CL, Hollis BW, Johnson DD. Plasma 25-hydroxyvitamin D levels in early-onset severe preeclampsia. Am J Obstet Gynecol. 2010;203(4):366 e1-6. [PubMed ID: 20692641]. [PubMed Central ID: PMC3192365]. https://doi.org/10.1016/j.ajog.2010.06.036.
-
14.
Davis LM, Chang SC, Mancini J, Nathanson MS, Witter FR, O'Brien KO. Vitamin D insufficiency is prevalent among pregnant African American adolescents. J Pediatr Adolesc Gynecol. 2010;23(1):45-52. [PubMed ID: 19643639]. https://doi.org/10.1016/j.jpag.2009.05.005.
-
15.
Leffelaar ER, Vrijkotte TG, van Eijsden M. Maternal early pregnancy vitamin D status in relation to fetal and neonatal growth: Results of the multi-ethnic Amsterdam born children and their Development cohort. Br J Nutr. 2010;104(1):108-17. [PubMed ID: 20193097]. https://doi.org/10.1017/S000711451000022X.
-
16.
Mehta S, Hunter DJ, Mugusi FM, Spiegelman D, Manji KP, Giovannucci EL, et al. Perinatal outcomes, including mother-to-child transmission of HIV, and child mortality and their association with maternal vitamin D status in Tanzania. J Infect Dis. 2009;200(7):1022-30. [PubMed ID: 19673647]. [PubMed Central ID: PMC2758703]. https://doi.org/10.1086/605699.
-
17.
Gale CR, Robinson SM, Harvey NC, Javaid MK, Jiang B, Martyn CN, et al. Maternal vitamin D status during pregnancy and child outcomes. Eur J Clin Nutr. 2008;62(1):68-77. [PubMed ID: 17311057]. [PubMed Central ID: PMC2629513]. https://doi.org/10.1038/sj.ejcn.1602680.
-
18.
Marjamaki L, Niinisto S, Kenward MG, Uusitalo L, Uusitalo U, Ovaskainen ML, et al. Maternal intake of vitamin D during pregnancy and risk of advanced beta cell autoimmunity and type 1 diabetes in offspring. Diabetologia. 2010;53(8):1599-607. [PubMed ID: 20369220]. https://doi.org/10.1007/s00125-010-1734-8.
-
19.
Grant WB, Soles CM. Epidemiologic evidence supporting the role of maternal vitamin D deficiency as a risk factor for the development of infantile autism. Dermatoendocrinol. 2009;1(4):223-8. [PubMed ID: 20592795]. [PubMed Central ID: PMC2835879]. https://doi.org/10.4161/derm.1.4.9500.
-
20.
Salzer J, Svenningsson A, Sundstrom P. Season of birth and multiple sclerosis in Sweden. Acta Neurol Scand. 2010;121(1):20-3. [PubMed ID: 19930210]. https://doi.org/10.1111/j.1600-0404.2009.01181.x.
-
21.
Bassir M, Laborie S, Lapillonne A, Claris O, Chappuis MC, Salle BL. Vitamin D deficiency in Iranian mothers and their neonates: A pilot study. Acta Paediatr. 2001;90(5):577-9. [PubMed ID: 11430721].
-
22.
Mulligan ML, Felton SK, Riek AE, Bernal-Mizrachi C. Implications of vitamin D deficiency in pregnancy and lactation. Am J Obstet Gynecol. 2010;202(5):429 e1-9. [PubMed ID: 19846050]. [PubMed Central ID: PMC3540805]. https://doi.org/10.1016/j.ajog.2009.09.002.
-
23.
Nowson CA, Margerison C. Vitamin D intake and vitamin D status of Australians. Med J Aust. 2002;177(3):149-52. [PubMed ID: 12149085].
-
24.
Prentice A. Micronutrients and the bone mineral content of the mother, fetus and newborn. J Nutr. 2003;133(5 Suppl 2):1693S-9S. [PubMed ID: 12730486]. https://doi.org/10.1093/jn/133.5.1693S.
-
25.
Specker B. Vitamin D requirements during pregnancy. Am J Clin Nutr. 2004;80(6 Suppl):1740S-7S. [PubMed ID: 15585798]. https://doi.org/10.1093/ajcn/80.6.1740S.
-
26.
Thomson K, Morley R, Grover SR, Zacharin MR. Postnatal evaluation of vitamin D and bone health in women who were vitamin D-deficient in pregnancy, and in their infants. Med J Aust. 2004;181(9):486-8. [PubMed ID: 15516192].
-
27.
Awad AB, Alappat L, Valerio M. Vitamin d and metabolic syndrome risk factors: Evidence and mechanisms. Crit Rev Food Sci Nutr. 2012;52(2):103-12. [PubMed ID: 22059957]. https://doi.org/10.1080/10408391003785458.
-
28.
Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr. 2004;79(5):820-5. [PubMed ID: 15113720]. https://doi.org/10.1093/ajcn/79.5.820.
-
29.
Seshadri KG, Tamilselvan B, Rajendran A. Role of vitamin D in diabetes. J Endocrinol Metab. 2011;1(47-56). https://doi.org/10.4021/jem23w.
-
30.
Poel YH, Hummel P, Lips P, Stam F, van der Ploeg T, Simsek S. Vitamin D and gestational diabetes: A systematic review and meta-analysis. Eur J Intern Med. 2012;23(5):465-9. [PubMed ID: 22726378]. https://doi.org/10.1016/j.ejim.2012.01.007.
-
31.
Scragg R, Sowers M, Bell C; Third National Health; Nutrition Examination Survey. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination survey. Diabetes Care. 2004;27(12):2813-8. [PubMed ID: 15562190]. https://doi.org/10.2337/diacare.27.12.2813.
-
32.
Dawodu A, Saadi HF, Bekdache G, Javed Y, Altaye M, Hollis BW. Randomized controlled trial (RCT) of vitamin D supplementation in pregnancy in a population with endemic vitamin D deficiency. J Clin Endocrinol Metab. 2013;98(6):2337-46. [PubMed ID: 23559082]. https://doi.org/10.1210/jc.2013-1154.
-
33.
Ryu OH, Lee S, Yu J, Choi MG, Yoo HJ, Mantero F. A prospective randomized controlled trial of the effects of vitamin D supplementation on long-term glycemic control in type 2 diabetes mellitus of Korea. Endocr J. 2014;61(2):167-76. [PubMed ID: 24240575]. https://doi.org/10.1507/endocrj.ej13-0356.
-
34.
Shakiba M, Iranmanesh MR. Vitamin D requirement in pregnancy to prevent deficiency in neonates: A randomised trial. Singapore Med J. 2013;54(5):285-8. [PubMed ID: 23716156]. https://doi.org/10.11622/smedj.2013110.
-
35.
Wagner CL, McNeil R, Hamilton SA, Winkler J, Rodriguez Cook C, Warner G, et al. A randomized trial of vitamin D supplementation in 2 community health center networks in South Carolina. Am J Obstet Gynecol. 2013;208(2):137 e1-13. [PubMed ID: 23131462]. [PubMed Central ID: PMC4365423]. https://doi.org/10.1016/j.ajog.2012.10.888.
-
36.
Witham MD, Dove FJ, Dryburgh M, Sugden JA, Morris AD, Struthers AD. The effect of different doses of vitamin D(3) on markers of vascular health in patients with type 2 diabetes: A randomised controlled trial. Diabetologia. 2010;53(10):2112-9. [PubMed ID: 20596692]. https://doi.org/10.1007/s00125-010-1838-1.
-
37.
Saedinea A, Larijani B, Jalalinea S, Farzadfar F, Keshtkar F, Rezae A. Evaloation of prevalence deficiency of vitamin D in Iranian population the province during 1990-2010. Iran J Diabetes Metab. 2013;12(6):574-84.
-
38.
Rahbar N, Rajabi M, Mirmohammadkhani M. 25-hydroxy vitamin D serum level in pregnant women with 8-12 gestational weeks in Semnan city and its association with fasting blood sugar and body mass index. Iran J Obstet Gynecol Infertil. 2015:1-8.
-
39.
Yap C, Cheung NW, Gunton JE, Athayde N, Munns CF, Duke A, et al. Vitamin D supplementation and the effects on glucose metabolism during pregnancy: A randomized controlled trial. Diabetes Care. 2014;37(7):1837-44. [PubMed ID: 24760259]. https://doi.org/10.2337/dc14-0155.
-
40.
Asemi Z, Hashemi T, Karamali M, Samimi M, Esmaillzadeh A. Effects of vitamin D supplementation on glucose metabolism, lipid concentrations, inflammation, and oxidative stress in gestational diabetes: A double-blind randomized controlled clinical trial. Am J Clin Nutr. 2013;98(6):1425-32. [PubMed ID: 24132976]. https://doi.org/10.3945/ajcn.113.072785.
-
41.
Asemi Z, Samimi M, Tabassi Z, Shakeri H, Esmaillzadeh A. Vitamin D supplementation affects serum high-sensitivity C-reactive protein, insulin resistance, and biomarkers of oxidative stress in pregnant women. J Nutr. 2013;143(9):1432-8. [PubMed ID: 23884390]. https://doi.org/10.3945/jn.113.177550.
-
42.
Soheilykhah S, Mojibian M, Moghadam MJ, Shojaoddiny-Ardekani A. The effect of different doses of vitamin D supplementation on insulin resistance during pregnancy. Gynecol Endocrinol. 2013;29(4):396-9. [PubMed ID: 23350644]. https://doi.org/10.3109/09513590.2012.752456.
-
43.
Althaus J. Vitamin D and pregnancy: 9 things you need to know. OBG Manag. 2011;23:30.
-
44.
Orwoll E, Riddle M, Prince M. Effects of vitamin D on insulin and glucagon secretion in non-insulin-dependent diabetes mellitus. Am J Clin Nutr. 1994;59(5):1083-7. [PubMed ID: 8172095]. https://doi.org/10.1093/ajcn/59.5.1083.
-
45.
Maghbooli Z, Hossein-Nezhad A, Karimi F, Shafaei AR, Larijani B. Correlation between vitamin D3 deficiency and insulin resistance in pregnancy. Diabetes Metab Res Rev. 2008;24(1):27-32. [PubMed ID: 17607661]. https://doi.org/10.1002/dmrr.737.
-
46.
Hosseinzadeh-Shamsi-Anar M, Mozaffari-Khosravi H, Salami MA, Hadinedoushan H, Mozayan MR. The efficacy and safety of a high dose of vitamin d in mothers with gestational diabetes mellitus: A randomized controlled clinical trial. Iran J Med Sci. 2012;37(3):159-65. [PubMed ID: 23115447]. [PubMed Central ID: PMC3470091].