1. Background
Diabetic retinopathy (DR) is one of the most severe eye complications caused by diabetes, leading to visual impairment and blindness (1). The DR is characterized by increased and dysregulation of blood glucose levels due to insulin resistance or decreased insulin production, affecting neovascularization in the retina (2, 3). In DR patients, the microvascular system is damaged in diabetes, especially in the retina and optic nerve. Furthermore, the hyperglycemic status in DR patients plays a dual role in the progression of nephrological diseases (e.g., albuminuria), along with DR, due to the preceded systemic inflammation (4). Therefore, numerous DR patients suffer from albuminuria (5-7).
Microalbuminuria and macroalbuminuria are two common forms of albuminuria characterized by increased urine albumin (8). Most albuminuria cases are defined by the urine albumin-to-creatinine ratio (ACR). The level of damage to the glomerulus identifies the type of albuminuria; the high permeability for albumin in the glomerulus and more ACR are the hallmarks of more damage (9). Microalbuminuria is more prevalent in DR patients, compared to macroalbuminuria (10).
The presence of long-term hyperglycemia creates a complex condition, including a chronic kidney disease (microalbuminuria), a chronic metabolic disease (diabetes), and a visual-impairing disease (retinopathy) (2, 8). Therefore, the management of a microalbuminuric DR patient needs dual considerations. The therapeutic procedure of microalbuminuric DR patients should contain diabetic restrictors, anti-neovascularization drugs, and renal-function management (3, 11).
In this regard, it is predicted that microalbuminuria status is correlated with DR indicators in terms of the glycemic status of patients. This study investigated the correlation of microalbuminuria indicators and DR indicators to evaluate the importance of microalbuminuria in DR patients.
2. Methods
In this study, 48 DR patients were admitted to the Ophthalmology Center of Ayatollah Rouhani Hospital affiliated with Babol University of Medical Sciences, Babol, Iran. Initial examinations were performed one hour after the administration of 2.5% phenylephrine and 0.5% tropicamide. Moreover, funduscopic examinations were carried out using a binocular indirect ophthalmoscope, 28D lens, scleral depressor, and speculum. In addition, the amount of visual acuity (VA) was measured. Furthermore, diabetic status was confirmed using laboratory tests (i.e., fast blood sugar [FBS] and hemoglobin A1c [HbA1c]). The inclusion criteria regarding diabetic status were simultaneous FBS > 120 mg/dL and HbA1c > 6% of total hemoglobin (Hb).
Then, the patients were treated with an intravitreal injection of 1.25 mg/0.05 mL bevacizumab (Avastin®) in the distance of 3-4-millimeter of the limbus (3 mm in aphakic and pseudophakic patients and 4 mm in phakic patients) under sterile conditions. Antibiotics were prescribed for a week, starting 3 days before the injection, for the prevention of unwanted infections. The second examination was conducted 45 days after the intervention. The FBS and HbA1c were evaluated using standard laboratory methods on the first day of bevacizumab intervention.
In addition, all statistical analyses were conducted using SPSS software (version 21). The independent sample t-test and Spearman test were used for statistical analysis. The significance level was considered 0.05 (confidence interval: 95%).
3. Results
3.1. Patient Characterization
In this follow-up study, 48 DR patients (24 male and 24 female subjects) participated with a mean age value of 57.87 ± 7.78 years. Moreover, 17 DR patients suffered from microalbuminuria, and 31 DR patients were healthy regarding microalbuminuria. The mean values of FBS and HbA1c were 176.72 ± 30.48 mg/dL and 9.38 ± 1.34 of total Hb.
3.2. Correlation Between Microalbuminuria and VA in DR Patients
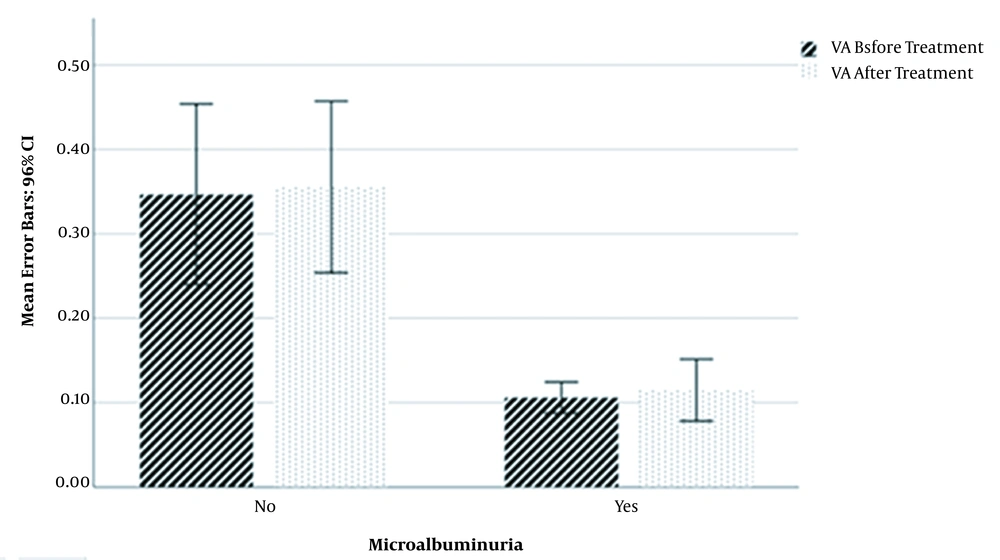
The mean value of VA before treatment in microalbuminuria patients (0.106 ± 0.036) was significantly lower than non-microalbuminuria patients (0.347 ± 0.286) (P < 0.001; T = -4.547). In addition, the VA mean value after treatment in microalbuminuria patients (0.115 ± 0.071) was significantly lower than non-microalbuminuria patients (0.355 ± 0.272) (P < 0.001; T = -4.578). There was no significant difference in the percentage of VA increase between microalbuminuria and non-microalbuminuria patients (Figure 1).
Mean Values of Visual Acuity Before and After Diabetic Retinopathy Treatment in Microalbuminuric and Non-microalbuminuric Diabetic Retinopathy Patients. Visual acuity values before and after treatment with bevacizumab were significantly higher in non-microalbuminuria diabetic retinopathy patients than microalbuminuria diabetic retinopathy patients.
3.3. Correlation Between ACR and VA in DR Patients
The current study results showed that a higher value of ACR was correlated with a lower level of VA before and after treatment (P < 0.001 for both) in DR patients. The microalbumin and creatinine levels were individually correlated with VA before and after treatment (P < 0.001 for both). However, there was no correlation between the percentage of VA increase with ACR, microalbumin, and creatinine.
3.4. Correlation of Microalbuminuria with FBS and HbA1c in DR Patients
The mean value of FBS in microalbuminuric DR patients (194.52 ± 13.78 mg/dL) was significantly higher than non-microalbuminuric DR patients (167.93 ± 32.91 mg/dL) (P < 0.001; T = 3.867). Furthermore, the mean value of HbA1c in microalbuminuric DR patients (10.45 ± 1.38) was significantly higher than non-microalbuminuric DR patients (8.83 ± 0.89) (P < 0.001; T = 4.363).
4. Discussion
Microalbuminuria is a defective glomerular condition that appears in different renal failures. Diabetes is also a risk factor for microalbuminuria condition. Additionally, diabetes leads to visual impairment defined as DR. The presence of microalbuminuria in DR patients is a common condition. In other words, DR patients usually suffer from renal failure (10, 12).
The present study results showed that the VA of DR patients before bevacizumab therapy was correlated with microalbuminuria indicators. The VA values before and after treatment in microalbuminuria patients were significantly lower than non-microalbuminuria patients (P < 0.001). This finding showed that VA is probably affected by the diabetic status of DR patients; however, microalbuminuria is also affected by diabetic status. In other words, diabetes is a risk factor for worse VA in DR patients and the presence of microalbuminuria.
Regarding the present study findings, various studies have investigated the correlation between microalbuminuria and DR. In 2004, Manaviat et al. revealed that microalbuminuria is associated with DR in type II diabetic patients. Moreover, in diabetic patients, HbA1c was significantly higher in DR patients than non-DR patients (13). Ucgul Atilgan et al. showed that central macular thickness is significantly higher in DR patients with microalbuminuria than diabetic patients without retinopathy and microalbuminuria (7). Lunetta et al. introduced microalbuminuria as a potent risk factor for retinopathy (14). In addition, the results of a study by Sobngwi et al. confirmed that microalbuminuria is a sensitive hallmark of DR (15). Overall, the results of the current study and other reports strongly confirm that worse visual condition of DR patients is observed in the presence of microalbuminuria. Therefore, the management of DR is more complicated in microalbuminuria patients due to less VA.
The present study results showed that the increase of VA in DR patients through bevacizumab therapy was not correlated with microalbuminuria status. Therefore, microalbuminuria could not be a bevacizumab therapy outcome predictor in DR patients. Furthermore, microalbuminuria could be an independent predicting factor for VA of DR patients. Therefore, the ACR should be checked for all DR patients to avoid the silent side effects of renal failure. There was no related study to evaluate the impact of microalbuminuria on bevacizumab therapy outcome; nevertheless, some studies established the role of microalbuminuria correlation with intravitreal bevacizumab therapy outcome in diabetic organopathies. For example, in a 2020 study performed by Hanna et al., intravitreal bevacizumab therapy was significantly correlated with more secretion of urine albumin in diabetic nephropathy (16).
Finally, the current study results showed that different VA before and after bevacizumab therapy status was correlated with microalbuminuria status; nonetheless, microalbuminuria status did not affect the percentage of VA increase (in comparison to those before and after bevacizumab therapy) in DR patients. It is suggested to perform further studies to investigate the impact of microalbuminuria on different DR therapeutic regimes with more participants. Moreover, the effect of other metabolic complications (e.g., hyperlipidemia) on the outcomes of the bevacizumab therapy regime can be studied in DR patients.
4.1. Conclusions
Microalbuminuria is a renal failure-related condition resulting in a primary disease (e.g., diabetes). The correlation between microalbuminuria and DR was previously established. The present study results confirmed that the VA of DR patients (before and after treatment) was significantly different in various microalbuminuria statuses. Additionally, it was revealed that microalbuminuria was not an effective factor in the outcomes of bevacizumab therapy of DR patients.