Abstract
Background:
Iron management is essential for anemia treatment in chronic kidney disease. The reticulocyte hemoglobin equivalent (RET-He) is a reticulocyte parameter that reflects hemoglobin synthesis of newly formed erythrocytes in the bone marrow in real-time.Objectives:
This study aims to evaluate the role of reticulocyte hemoglobin equivalent (RET-He) in predicting iron deficiency in chronic kidney disease (CKD) patients.Methods:
Following a descriptive cross-sectional observational design, this study was conducted on 131 adult patients with CKD stages 3 - 5. Laboratory indices, including complete blood count, some biochemical indices, iron status, and reticulocyte indices (including RET-He), were measured. Iron deficiency (ID) was defined as TSAT < 20%, where serum ferritin level > 100 ng/mL was defined as functional ID, while serum ferritin level <100 ng/mL was defined as absolute ID.Results:
Nearly 42% of patients had ID. The mean concentration of RET-He in CKD patients with ID was significantly lower than that of patients without ID (P < 0.001). Based on the Receiver Operating Characteristic (ROC) curve model, RET-He had a good predictive value for ID in CKD patients (AUC = 0.762; P < 0.001; cut-off value: 28.15 g/L, the sensitivity of 45.5%, and the specificity of 100%). Serum iron, RET-He, serum albumin, and mean corpuscular volume (MCV) were independent risk factors to predict ID in CKD patients.Conclusions:
This study demonstrated that RET-He is an appropriate index to predict ID in CKD patients.Keywords
1. Background
Chronic kidney disease (CKD) is a widespread health problem affecting millions worldwide. CKD not only is associated with increased incidence, prevalence, and cost of treatment but also leads to end-stage CKD. Anemia is a common complication of CKD that contributes to reduced quality of life and poor treatment outcomes (1, 2). Erythropoiesis-stimulating agents (ESAs) promote erythropoiesis in CKD patients; however, iron deficiency often leads to decreased response to treatment with ESA (3). Iron deficiency often leads to increased doses of ESAs, causing adverse effects in patients (4). Therefore, the assessment of iron status in CKD patients is essential. In clinical practice, clinicians often use traditional markers to evaluate iron status, such as serum iron, ferritin, and transferrin saturation (TSAT) levels (5). Reticulocyte hemoglobin equivalent (RET-He) is a new indicator to assess the iron status of immature erythrocytes, which can be measured by a flow cytometry technique (4). RET-He is a parameter that reflects hemoglobin synthesis by newly formed red blood cells in the bone marrow in real-time. In order to clarify the role of RET-He in predicting ID in CKD patients, we conducted this study to "evaluate the role of Reticulocyte hemoglobin equivalent (RET-He) for predicting iron deficiency in chronic kidney disease patients."
2. Objectives
This study aimed to evaluate the RET-He role in predicting iron deficiency in CKD patients.
3. Methods
Following a descriptive cross-sectional observational design, this study was conducted on 131 adult patients with CKD stages 3 - 5 attending the Department of Nephrology and Hemodialysis, Military Hospital 103, from October 2019 to October 2020. Patients aged 16 years and above, in both genders, diagnosis with CKD stages 3 - 5 (without undergoing hemodialysis) who were willing to participate were recruited in this study. We excluded patients who were on renal replacement therapy, or had a history of receiving EPO within the previous two weeks, or had a history of receiving iron therapy, and those with evidence of bleeding, infection, or blood transfusion within the past six months.
Laboratory investigation included complete blood count, conventional biochemical indices, high-sensitivity C-reactive protein (hs-CRP), free iron, and ferritin concentration tests. CKD was diagnosed and staged based on the National Kidney Foundation/Kidney Disease Outcome Quality Initiative (NKF/KDOQI) criteria (6). Anemia in CKD was defined as World Health Organization (WHO) criteria (Hb < 130 g/L in males and < 120 g/L in females) (7). Serum iron profiles consist of free iron, ferritin, and TIBC, in which TIBC was estimated by a Diagnosis-Related Group TIBC (bioactive) enzyme-linked immunosorbent assay kit using the principle of competitive binding.
TSAT (%) was calculated as ((serum iron × 100)/TIBC). Iron deficiency (ID) was defined as TSAT < 20%, where serum ferritin level > 100 ng/mL was defined as functional ID, while serum ferritin level < 100 ng/mL was defined as absolute ID, according to the NKF's KDOQI anemia guidelines (8).
Complete blood count and reticulocyte indices were performed on a Sysmex XN1000 analyzer, Japan. RET-He was measured in the reticulocyte channel by flow cytometry using a Polymerthin fluorescent dye.
3.1. Statistical Analysis
All the continuous data were represented by the mean and standard deviation (for normally distributed data) or by the median and interquartile (for non-normally distributed data). These data were compared by the Student's t-test or Mann-Whitney U test, respectively. Categorical data were presented by the frequency with percentage and were analyzed using the Chi-square test. Pearson correlation was used to measure the degree of the association between linearly related variables. The receiver operating characteristic (ROC) curves with the area under the curve (AUC) were calculated to predict ID. Multivariate adjusted regression analysis was performed to determine the independent risk factors for ID (using a backward selection procedure). Statistical analysis was calculated by the Statistical Package for the Social Science version 22.0. A P-value < 0.05 was considered significant.
4. Results
Table 1 shows the baseline characteristics of all patients. The mean age of all patients was 51.56 ± 18.2 years, in which the proportion of males was more than 70%. There were 51 patients with CKD stages 3 - 4 in our study, and 80 patients had CKD stage 5. In our study, the proportion of hypertension, anemia, and ID were 84%, 93.1%, and 42%, respectively. The mean RET-He concentration was 30.2 ± 3.69 g/L. When comparing the demographic and laboratory characteristics between the two groups (ie, CKD stages 3 - 4 and CKD stage 5), we found no difference in some baseline characteristics such as the mean age, gender, body mass index (BMI), anemia, as well as some laboratory indices such as MCV, MCH, MCHC, absolute reticulocyte counts, and serum iron concentration. Besides, as shown in Table 1, patients with CKD stage 5 had a significantly higher proportion of hypertension, CGN etiology, moderate, and severe anemia; higher median concentration of urea, creatinine, hs-CRP, reticulocyte percentage, RET-He, serum ferritin, and TSAT; lower eGFR, serum albumin level, TIBC, and proportion of ID, as compared to those of patients with CKD stages 3 - 4.
Clinical Characteristics and Laboratory Parameters for Each Chronic Kidney Disease Stage (N = 131) a
| Clinical Characteristics and Laboratory Parameters | All Patients (N = 131) | CKD Stage 3 and Stage 4 (N = 51) | CKD Stage 5 (N = 80) | P-Value |
|---|---|---|---|---|
| Ages (y) | 51.56 ± 18.2 | 53.71 ± 18.11 | 50.19 ± 18.24 | 0.283 |
| Sex | 0.419 | |||
| Female | 36 (27.5) | 12 (23.5) | 24 (30) | |
| Male | 95 (72.5) | 39 (76.5) | 56 (70) | |
| BMI | 20.34 ± 1.94 | 20.48 ± 1.95 | 20.24 ± 1.93 | 0.496 |
| Hypertension | 110 (84) | 36 (70.6) | 74 (92.5) | 0.001 |
| Etiology | 0.001 | |||
| CGN | 62 (47.3) | 15 (29.4) | 47 (58.8) | |
| Chronic pyelonephritis | 24 (18.3) | 15 (29.4) | 9 (11.3) | |
| Diabetes | 19 (14.5) | 9 (17.6) | 10 (12.5) | |
| Hypertension | 20 (15.3) | 12 (23.5) | 8 (10) | |
| Polycystic kidney diseases | 6 (4.6) | 0 (0) | 6 (7.5) | |
| Serum Urea (mmol/L) | 23.8 (15.3 - 35.4) | 13.3 (9.8 - 17.5) | 31.6 (24.94 - 41.2) | < 0.001 |
| Serum creatinine (µmol/L) | 503 (265 - 866) | 201 (165 - 294) | 752 (577.25 - 1000.25) | < 0.001 |
| eGFR (ml/mim/1.73 m2) | 10.5 (5.7 - 20.4) | 28.9 (16.9 - 35.9) | 6.5 (5.02 - 9.5) | < 0.001 |
| Serum albumin (g/L) | 35.93 ± 6.12 | 37.43 ± 5.73 | 34.97 ± 6.21 | 0.024 |
| hs-CRP (mg/L) | 2.5 (0.5 - 11.8) | 1.08 (0.32 - 3.25) | 3.1 (1.1 - 14.87) | 0.003 |
| Anemia | 122 (93.1) | 45 (88.2) | 77 (96.3) | 0.153 |
| Severity of anemia | < 0.001 | |||
| Mild | 41 (33.6) | 27 (60) | 14 (18.2) | |
| Moderate | 46 (37.7) | 15 (33.3) | 31 (40.3) | |
| Severe | 35 (28.7) | 3 (6.7) | 32 (41.6) | |
| Hb (g/L) | 96.01 ± 26.45 | 107.88 ± 29.4 | 88.44 ± 21.33 | < 0.001 |
| MCV (fL) | 85.05 ± 8.01 | 83.35 ± 9.17 | 86.13 ± 7.02 | 0.069 |
| MCH (pg) | 27.93 ± 3.19 | 27.44 ± 3.48 | 28.25 ± 2.98 | 0.177 |
| MCHC (g/L) | 324.17 ± 22.33 | 321.56 ± 19.09 | 325.83 ± 24.14 | 0.288 |
| RET# (T/l) | 0.071 (0.062 - 0.084) | 0.071 (0.062 - 0.075) | 0.073 (0.064 - 0.085) | 0.771 |
| RET% (%) | 2.00 ± 0.65 | 1.84 ± 0.5 | 2.1 ± 0.72 | 0.032 |
| RET-He (g/L) | 30.2 ± 3.69 | 29.11 ± 4.15 | 30.9 ± 3.2 | 0.01 |
| Serum iron (µmol/L) | 10.8 (6.6 - 15.1) | 10.8 (7.2 - 15.1) | 10.85 (6.45 - 14.9) | 0.538 |
| Ferritin (ng/mL) | 369.37 (173.02 - 488.28) | 252.6 (187.1 - 405.65) | 404.38 (169.38 - 541.02) | 0.041 |
| TIBC (µmol/L) | 51.47 (39.57 - 72.3) | 72.3 (67.9 - 81.25) | 43.38 (35.09 - 59.62) | < 0.001 |
| TSAT (%) | 20.58 (12.73 - 24.36) | 17.05 (10.33 - 20.58) | 22.46 (18.05 - 31.96) | < 0.001 |
| Iron deficiency | 0.002 | |||
| Yes | 55 (42) | 30 (58.8) | 25 (31.2) | |
| No | 76 (58) | 21 (41.2) | 55 (68.8) |
When comparing some hematological indices based on ID status, we found that the mean concentration of MCHC and RET-He in CKD patients with ID was significantly lower than that of patients without ID (P = 0.001 and P < 0.001, respectively). CKD patients with ID had lower hemoglobin, MCH, MCHC, absolute reticulocyte count, and a higher reticulocyte percentage. However, the difference is not statistically significant, with P > 0.05 (Table 2).
Comparison of Some Hematological Indices Based on Iron Deficiency Status (N = 131)
| Variables | Iron Deficiency (N = 55) | None (N = 76) | P-Value |
|---|---|---|---|
| Hb (g/L) | 94.44 ± 25.66 | 97.15 ± 27.12 | 0.565 |
| MCV (fL) | 84.75 ± 8.21 | 85.26 ± 7.91 | 0.719 |
| MCH (pg) | 27.67 ± 2.58 | 28.12 ± 3.58 | 0.432 |
| MCHC (g/L) | 316.89 ± 16.84 | 329.44 ± 24.35 | 0.001 |
| RET# (T/l) | 0.071 (0.058 - 0.087) | 0.073 (0.065 - 0.084) | 0.325 |
| RET% (%) | 2.01 ± 0.81 | 1.99 ± 0.51 | 0.918 |
| RET-He (g/L) | 27.99 ± 4.18 | 31.8 ± 2.2 | < 0.001 |
As shown in Table 3, there was a strong positive correlation between RET-He and serum iron (r = 0.513, P < 0.001), and TSAT (r = 0.589, P < 0.001); a moderate positive correlation between RET-He and Ferritin (r = 0.399, P < 0.001); and a weak negative correlation between RET-He and TIBC (r = -0.182, P = 0.037) (Table 3).
Correlation Between RET-He and Several Iron Status Indices (N = 131)
| Indices | RET-He (g/L) | Correlation Equation | |
|---|---|---|---|
| r | P-Value | ||
| Serum iron (µmol/L) | 0.513 | < 0.001 | RET-He = 0.299 × Serum iron + 26.746 |
| Ferritin (ng/mL) | 0.399 | < 0.001 | RET-He = 0.008 × Ferritin + 27.522 |
| TIBC (µmol/L) | -0.182 | 0.037 | RET-He = 32.139 - 0.034 × TIBC |
| TSAT (%) | 0.589 | < 0.001 | RET-He = 0.166 × TSAT + 26.529 |
Based on the ROC curve model, RET-He had a good predictive value of ID in CKD patients compared to some traditional iron indices (AUC = 0.762; P < 0.001; cut-off value: 28.15g/L, the sensitivity of 45.5%, and the specificity of 100%) (Figure 1).
Receiver operating characteristics (ROC) curves of RET-He and some iron status indices for prediction of iron deficiency in CKD patients (n = 131). RET-He: AUC = 0.762; P < 0.001; Cut-off: 28.15 (g/L); Sensitivity: 45.5%; Specificity: 100%
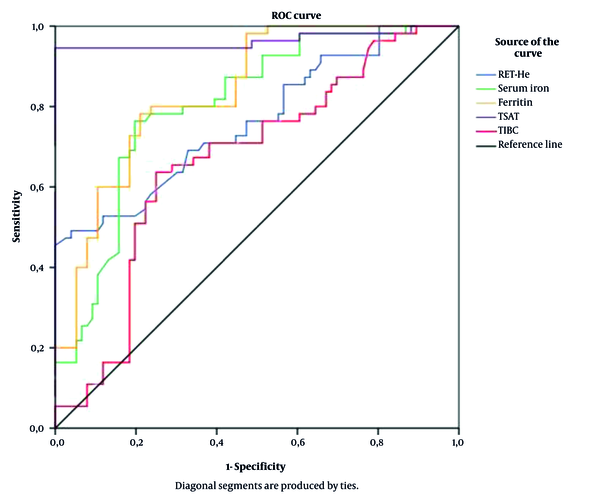
Table 4 shows that serum iron, RET-He, serum albumin, and MCV are independent risk factors for ID in CKD patients.
Multivariate Logistic Regression Analysis Concerning Prognosis Factor for Iron Deficiency in CKD Patients (N = 131)
| Variables | OR | 95% Cl | P-Value |
|---|---|---|---|
| Serum iron (µmol/L) | 0.833 | 0.741 - 0.936 | 0.002 |
| RET-He (g/L) | 0.624 | 0.482 - 0.81 | < 0.001 |
| Albumin (g/L) | 1.126 | 1.028 - 1.232 | 0.011 |
| MCV (fL) | 1.074 | 1.003 - 1.149 | 0.04 |
| MCHC (g/L) | 0.97 | 0.938 - 1.003 | 0.076 |
5. Discussion
We apply the WHO diagnostic criteria for anemia based on hemoglobin concentration (HGB). Anemia was defined as HGB < 130 g/L for adult men and < 120 g/L for women. In our study, the proportion of anemia was 93.1% (patients with CKD stages 3 and 4 were 88.2%, and patients with CKD stage 5 were 96.3%). The prevalence of anemia in our study was higher than in some other studies. Li et al. studied the proportion and severity of anemia in 2420 CKD patients in Shanghai, China, and indicated that the proportion of anemia in patients with chronic kidney disease (CKD) stages 3, 4, and 5 was 51.1%, 79.2%, and 90.2%, respectively (9). Salman et al. showed that the proportion of anemia was 75.8% in all stages. The proportion of anemia in CKD stage 3 was 41.9%, stage 3b was 63.4%, stage 4 was 85.4%, and stage 5 was 97.4% (10). Anemia is a widespread complication in CKD patients with and without kidney failure.
The prevalence of anemia usually increases with the CKD stage. The more severe the renal function is, the higher the proportion of anemia and its severity. This reflects the pathogenesis of chronic renal failure. As the kidney function decreases drastically, the severity of metabolic and endocrine disorders also increases, in which the disturbance of erythropoietin secretion worsens (10-12). In addition, inflammatory cytokines inhibit hematopoiesis in the bone marrow due to the development of alkaline balance disorder and increased levels of free radicals, which may worsen anemia (13). Many subsequent studies provided evidence to better understand the causes and mechanisms of anemia in CKD patients more fully. All three mechanisms of anemia had been seen in CKD patients, including reduced hematopoiesis in bone marrow, increased destruction of red blood cells, and blood loss (14).
Since the KDOQI 2006 workgroup recommended targeting serum ferritin levels of more than 100 ng/mL in patients with non-dialysis ESRD, some randomized control studies showed that iron treatment had erythropoietic effects in patients with stages 3 – 5 CKD and ferritin levels exceeding 100 ng/mL (8, 15). Currently, the Vietnamese Nephrology Association covers the use of intravenous iron in patients with CKD and with TSAT < 20.0% or ferritin < 100 ng/mL, and Hb lower than 10 g/dL. In our study, the total ID proportion was 42%. When investigating iron status in CKD patients, we found that patients with CKD stage 5 had a significantly lower proportion of ID than patients with CKD stages 3 - 4 (58.8% compared to 32.2%). This result can be attributed to the fact that most patients with CKD stages 3 - 4 were newly discovered and had no history of receiving treatment. Therefore, the proportion of ID was higher than patients with stage 5 CKD who received treatment. Alzaheb and Al-Amer reported that ID anemia was prevalent (12.5%) among female university students in Saudi Arabian. They also reported that inadequate iron intake was a risk factor for anemia (16). In our study, the median concentration of serum ferritin and TSAT were significantly lower, and the median concentration of TIBC was significantly higher in patients with CKD stage 5 than stages 3 - 4. It can be attributed to the fact that in patients with chronic renal failure, ID can be caused by several factors such as inadequate iron intake due to poor diet or restrictive diets, reduced iron absorption, and blood loss through heavy periods or internal bleeding (17, 18). Several biochemical indices, such as serum ferritin concentration and TSAT, are used to diagnose ID. However, these tests also have certain restrictions: TSAT% is strongly affected by the daily iron fluctuation, while serum ferritin can be increased in chronic inflammatory diseases (19, 20). Therefore, RET-He has become a potential indicator to replace ferritin and TSAT to assess iron storage in CKD patients because it directly evaluates the iron used for the hemoglobin biosynthesis throughout erythropoiesis (21).
As results in Table 1 indicate, the mean value of the reticulocyte percentage (RET%) was 2%; absolute reticulocyte count (RET#) was 0.071 T/L, and reticulocyte hemoglobin equivalent (RET-He) was 30.2 pg. In our study, both percentage and the absolute value of reticulocytes were higher than those of Maconi et al., who studied over 200 dialysis patients (1.45% and 0.05 T/L, respectively). However, the concentration of RET-He in our study was lower than that of Maconi et al. (30.2 pg compared to 33.9 pg) (22). Miwa et al. studied 217 hemodialysis patients and showed that the RET‐He mean value was 32.4pg (23).
The observed difference in the findings of these studies can be attributed to the characteristics of participants. In our study, patients had not been treated with any renal replacement therapy or iron supplementation treatment. Therefore the proportion of patients with iron deficiency anemia in our study were higher than those above studies.
As shown in Table 2, the mean concentration of MCHC and RET-He in CKD patients with ID was significantly lower than that of patients without ID (P = 0.001 and P < 0.001, respectively). This finding is entirely consistent with the characteristic of iron-deficiency anemia, hypochromic anemia, and decreased concentration of RET-He.
When studying the correlation between RET-He and some iron status indices, we found a strong positive correlation between RET-He and serum iron (r = 0.513, P < 0.001), and TSAT (r = 0.589, P < 0.001); a moderate positive correlation between RET-He and Ferritin (r = 0.399, P < 0.001); and a slight negative correlation between RET-He and TIBC (r = -0.182, P = 0.037) (Table 3). Measurement of reticulocyte hemoglobin equivalent (RET-He) is a test to diagnose and monitor ID anemia. RET-He is an early measurement of the current iron supply to erythropoiesis in the bone marrow (24). Therefore, when ID occurs, manifesting in the depletion of red blood cells (decreased MCH) or decreased serum iron and ferritin concentrations, the concentration of RET-He will also decreases.
Many studies used RET-He as a reliable indicator to assess ID in CKD patients. Based on the ROC curve analysis (Figure 1), RET-He had a good predictive value for ID in CKD patients compared to some traditional iron indices (AUC = 0.762; P < 0.001; cut-off value: 28.15 g/L, the sensitivity of 45.5%, and the specificity of 100%). Our results were similar to those of Miwa et al.. In this study, RET-He had a good predicting value for ID with AUC = 0.776; cut-off value = 33pg; sensitivity = 74.3%; specificity = 64.9% (23). Brugnara et al. also showed that at the cut-off level of 27.2 pg, RET-He had a diagnostic value for ID with AUC = 0,913; P < 0.0001; sensitivity up to 93.3%; specificity of 83.2% (25). A multivariate regression model using backward selection criteria was established to confirm the predictive value of RET-He in predicting ID in CKD patients. The results presented in Table 4 show that serum iron, RET-He, serum albumin, and MCV were independent risk factors for predicting ID in CKD patients.
5.1. Conclusions
In conclusion, RET-He had a good predictive value of iron deficiency in CKD patients compared to some traditional iron indices.
References
-
1.
Eknoyan G, Lameire N, Barsoum R, Eckardt KU, Levin A, Levin N, et al. The burden of kidney disease: improving global outcomes. Kidney Int. 2004;66(4):1310-4. [PubMed ID: 15458424]. https://doi.org/10.1111/j.1523-1755.2004.00894.x.
-
2.
Nurko S. Anemia in chronic kidney disease: causes, diagnosis, treatment. Cleve Clin J Med. 2006;73(3):289-97. [PubMed ID: 16548452]. https://doi.org/10.3949/ccjm.73.3.289.
-
3.
Covic A, Nistor I, Donciu MD, Dumea R, Bolignano D, Goldsmith D. Erythropoiesis-stimulating agents (ESA) for preventing the progression of chronic kidney disease: a meta-analysis of 19 studies. Am J Nephrol. 2014;40(3):263-79. [PubMed ID: 25323019]. https://doi.org/10.1159/000366025.
-
4.
Hayes W. Measurement of iron status in chronic kidney disease. Pediatr Nephrol. 2019;34(4):605-13. [PubMed ID: 29666917]. [PubMed Central ID: PMC6394676]. https://doi.org/10.1007/s00467-018-3955-x.
-
5.
Bermejo F, Garcia-Lopez S. A guide to diagnosis of iron deficiency and iron deficiency anemia in digestive diseases. World J Gastroenterol. 2009;15(37):4638-43. [PubMed ID: 19787826]. [PubMed Central ID: PMC2754511]. https://doi.org/10.3748/wjg.15.4638.
-
6.
National Kidney F. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1-266. [PubMed ID: 11904577].
-
7.
World Health Organization. Haemoglobin for the diagnosis of anemia and assessment of severity: Vitamin and Mineral Nutrition Information System. World Health Organ. 2011:1-6.
-
8.
Kdoqi. KDOQI Clinical Practice Guideline and Clinical Practice Recommendations for anemia in chronic kidney disease: 2007 update of hemoglobin target. Am J Kidney Dis. 2007;50(3):471-530. [PubMed ID: 17720528]. https://doi.org/10.1053/j.ajkd.2007.06.008.
-
9.
Li Y, Shi H, Wang WM, Peng A, Jiang GR, Zhang JY, et al. Prevalence, awareness, and treatment of anemia in Chinese patients with nondialysis chronic kidney disease: First multicenter, cross-sectional study. Medicine (Baltimore). 2016;95(24). e3872. [PubMed ID: 27310973]. [PubMed Central ID: PMC4998459]. https://doi.org/10.1097/MD.0000000000003872.
-
10.
Salman M, Khan AH, Adnan AS, Sulaiman SA, Hussain K, Shehzadi N, et al. Prevalence and management of anemia in pre-dialysis Malaysian patients: A hospital-based study. Rev Assoc Med Bras (1992). 2016;62(8):742-7. [PubMed ID: 27992014]. https://doi.org/10.1590/1806-9282.62.08.742.
-
11.
Ryu SR, Park SK, Jung JY, Kim YH, Oh YK, Yoo TH, et al. The Prevalence and Management of Anemia in Chronic Kidney Disease Patients: Result from the KoreaN Cohort Study for Outcomes in Patients With Chronic Kidney Disease (KNOW-CKD). J Korean Med Sci. 2017;32(2):249-56. [PubMed ID: 28049235]. [PubMed Central ID: PMC5219990]. https://doi.org/10.3346/jkms.2017.32.2.249.
-
12.
Akizawa T, Makino H, Matsuo S, Watanabe T, Imai E, Nitta K, et al. Management of anemia in chronic kidney disease patients: baseline findings from Chronic Kidney Disease Japan Cohort Study. Clin Exp Nephrol. 2011;15(2):248-57. [PubMed ID: 21234785]. https://doi.org/10.1007/s10157-010-0396-7.
-
13.
Laurencet F, Balducci L, Ershler W, de Gaetano G. Qualitative changes of hematopoiesis. Blood Disorders in the Elderly. Cambridge University Press; 2008. p. 95-119. https://doi.org/10.1017/cbo9780511545238.009.
-
14.
Nangaku M, Eckardt KU. Pathogenesis of renal anemia. Semin Nephrol. 2006;26(4):261-8. [PubMed ID: 16949463]. https://doi.org/10.1016/j.semnephrol.2006.06.001.
-
15.
Qunibi WY, Martinez C, Smith M, Benjamin J, Mangione A, Roger SD. A randomized controlled trial comparing intravenous ferric carboxymaltose with oral iron for treatment of iron deficiency anaemia of non-dialysis-dependent chronic kidney disease patients. Nephrol Dial Transplant. 2011;26(5):1599-607. [PubMed ID: 20929915]. [PubMed Central ID: PMC3084440]. https://doi.org/10.1093/ndt/gfq613.
-
16.
Alzaheb RA, Al-Amer O. The Prevalence of Iron Deficiency Anemia and its Associated Risk Factors Among a Sample of Female University Students in Tabuk, Saudi Arabia. Clin Med Insights Womens Health. 2017;10:1179562X17745088. [PubMed ID: 29225484]. [PubMed Central ID: PMC5714083]. https://doi.org/10.1177/1179562X17745088.
-
17.
Babitt JL, Lin HY. Molecular mechanisms of hepcidin regulation: implications for the anemia of CKD. Am J Kidney Dis. 2010;55(4):726-41. [PubMed ID: 20189278]. [PubMed Central ID: PMC2905036]. https://doi.org/10.1053/j.ajkd.2009.12.030.
-
18.
Zumbrennen-Bullough K, Babitt JL. The iron cycle in chronic kidney disease (CKD): from genetics and experimental models to CKD patients. Nephrol Dial Transplant. 2014;29(2):263-73. [PubMed ID: 24235084]. [PubMed Central ID: PMC3910345]. https://doi.org/10.1093/ndt/gft443.
-
19.
Beard JL, Murray-Kolb LE, Rosales FJ, Solomons NW, Angelilli ML. Interpretation of serum ferritin concentrations as indicators of total-body iron stores in survey populations: the role of biomarkers for the acute phase response. Am J Clin Nutr. 2006;84(6):1498-505. [PubMed ID: 17158435]. https://doi.org/10.1093/ajcn/84.6.1498.
-
20.
Buttarello M, Pajola R, Novello E, Rebeschini M, Cantaro S, Oliosi F, et al. Diagnosis of iron deficiency in patients undergoing hemodialysis. Am J Clin Pathol. 2010;133(6):949-54. [PubMed ID: 20472854]. https://doi.org/10.1309/AJCPQAX0JFHFS0OA.
-
21.
Eguchi A, Mochizuki T, Tsukada M, Kataoka K, Hamaguchi Y, Oguni S, et al. Serum hepcidin levels and reticulocyte hemoglobin concentrations as indicators of the iron status of peritoneal dialysis patients. Int J Nephrol. 2012;2012:239476. [PubMed ID: 23193472]. [PubMed Central ID: PMC3501962]. https://doi.org/10.1155/2012/239476.
-
22.
Maconi M, Cavalca L, Danise P, Cardarelli F, Brini M. Erythrocyte and reticulocyte indices in iron deficiency in chronic kidney disease: comparison of two methods. Scand J Clin Lab Invest. 2009;69(3):365-70. [PubMed ID: 19125368]. https://doi.org/10.1080/00365510802657673.
-
23.
Miwa N, Akiba T, Kimata N, Hamaguchi Y, Arakawa Y, Tamura T, et al. Usefulness of measuring reticulocyte hemoglobin equivalent in the management of haemodialysis patients with iron deficiency. Int J Lab Hematol. 2010;32(2):248-55. [PubMed ID: 19624802]. https://doi.org/10.1111/j.1751-553X.2009.01179.x.
-
24.
Dalimunthe NN, Lubis AR. Usefulness of Reticulocyte Hemoglobin Equivalent in Management of Regular Hemodialysis Patients with Iron Deficiency Anemia. Rom J Intern Med. 2016;54(1):31-6. [PubMed ID: 27141568]. https://doi.org/10.1515/rjim-2016-0003.
-
25.
Brugnara C, Schiller B, Moran J. Reticulocyte hemoglobin equivalent (Ret He) and assessment of iron-deficient states. Clin Lab Haematol. 2006;28(5):303-8. [PubMed ID: 16999719]. [PubMed Central ID: PMC1618805]. https://doi.org/10.1111/j.1365-2257.2006.00812.x.