1. Background
Current studies suggest using cystatin C for a more accurate estimated glomerular filter rate (eGFR) (1-3). Cystatin C is a 13 kDa cysteine proteinase inhibitor protein secreted by all eukaryotes. It is filtered in the kidney membranes, reabsorbed near-completely, and catabolized in the proximal tubular. Until it was found as a glomerular filtration marker in 1985, its clinical application in comparison to creatinine was limited and argued. The methods and reagents were not standardized for determining cystatin C. That might cause bias in the results of cystatin C and the eGFR (4, 5). Based on the results of some studies, the classification of the chronic kidney disease (CKD) stages has been changed because of changes in cystatin C values in groups of age (6, 7). In Vietnam, despite the increasing rate of CKD, the clinical application of cystatin C is still small.
2. Objectives
This study aimed to estimate the concentration of cystatin C and its variations in different stages of CKD.
3. Methods
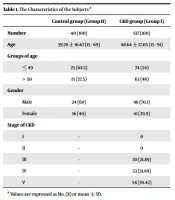
A cross-sectional study was done from December 2020 to June 2021 in two groups of subjects in 103 Hospital, Hanoi, Vietnam. Here, 137 patients with CKD at four stages were enrolled in group I (CKD group) and 40 healthy persons in group II (control group). The characteristics of the subjects are presented in Table 1. The heparinized blood samples were collected in the morning. Then they were centrifuged at 4000 rpm to collect plasma. The plasma was stored at -20°C. Cystatin C was determined in plasma by the Abbott reagent in the Architect Ci16200. The CKD was diagnosed and classified based on the KDIGO criterion.
| Control Group (Group II) | CKD Group (Group I) | |
|---|---|---|
| Number | 40 (100) | 137 (100) |
| Age | 39.78 ± 16.67 (15 - 69) | 48.64 ± 17.05 (15 - 91) |
| Groups of age | ||
| ≤ 49 | 25 (62.5) | 74 (54) |
| > 50 | 15 (37.5) | 63 (46) |
| Gender | ||
| Male | 24 (60) | 96 (70.1) |
| Female | 16 (40) | 41 (29.9) |
| Stage of CKD | ||
| I | - | 0 |
| II | - | 0 |
| III | - | 30 (21.89) |
| IV | - | 53 (31.69) |
| V | - | 54 (39.42) |
a Values are expressed as No. (%) or mean ± SD.
3.1. Statistical Analyses
Normally distributed variables are presented in mean ± standard deviation (SD). t-test was used to compare the differences between the mean of two distributed variable groups. The differences between the mean of more than two groups were assessed by one-way ANOVA test. Statistical significance was defined at P < 0.05. Statistical analyses were performed with SPSS 16.0 software.
4. Results
4.1. Cystatin C Levels in the Subjects
Cystatin C levels of the CKD group (group I) were significantly higher than the control group (group II) with P = 0.000 < 0.05 (9.17 ± 3.75 mg/L versus 0.82 ± 0.12 mg/L, respectively). In the CKD group (group I). The level of cystatin C increased linearly with the serious failure of the kidney. The groups at the next stages had a significantly higher Cystatin C level (groups of stage III, IV, and V with 4.85 ± 1.29; 7.39 ± 1.0; 13.32 ± 1.68, respectively) (Table 2).
a The control group versus the CKD group (t-test).
b The differences between three groups of three stages of CKD (stage III, IV, and V) (ANOVA test).
4.2. The Relationship Between the Cystatin C Level and the Gender
There were no differences in the cystatin C levels between males and females in the CKD group, in the three stages of CKD group, and in the control group. In the control group, the mean cystatin C levels of females and males was 0.78 ± 0.13 and 0.84 ± 0.11, respectively. In the CKD, the mean cystatin C levels of females and males was 8.75 ± 3.32 and 9.36 ± 3.91, respectively (Table 3).
a The male versus female in the control group.
b The male versus female in CKD group.
c The male versus female in stage III of CKD.
d The male versus the female in the stage IV of CKD.
e The male versus female in the stage V of CKD.
4.3. The Relationship Between the Cystatin C Level and Age
The subjects in this study were divided into the following age groups: Below 50 years and older than 50 years. There were no differences between the two groups of age in both control and CKD groups. In the CKD group, no differences in two periods of age were found in the three stages of the disease (Table 4).
a The age ≤ 49 versus the age ≥ 50 in the control group
b The age ≤ 49 versus the age ≥ 50 in the CKD group
c The age ≤ 49 versus the age ≥ 50 in the stage III CKD group
d The age ≤ 49 versus the age ≥ 50 in the stage IV CKD group
e The age ≤ 49 versus the age ≥ 50 in the stage V CKD group
5. Discussion
The current study was conducted on 137 patients with CKD stages III, IV, and V and 40 healthy controls at military hospital 103. The mean age of healthy patients was 39.78 ± 16.67 years, and the group of patients with CKD was 48.64 ± 17.05 years. The mean age of the subjects in the present study was younger than others studies (8). Many studies have suggested the differences at ages 50, 70, and 80 years (7, 9). All subjects of this study were randomly chosen, and the mean age was young. This problem shows that the subset of the CKD might occur at a younger age in Vietnam. The demand for early detection is necessary to decrease the disease and the cost of the treatment.
Cystatin C levels of the healthy Vietnamese people in the control group were similar to some researches in the world. The mean cystatin C level in the CKD group was different. A study by Zati Iwani et al. on 418 normal people and patients showed that the concentration of normal people’s cystatin C, as well as stage 1, 2, and 3 of CKD were 0.8 ± 0.2 mg/L, 0.8 ± 0.2 mg/L, 1.0 ± 0.3 mg/L, 1.3 ± 0.4 mg/L respectively (10). Furthermore, there was a difference between the concentration of the three stages with P < 0.01 (10). A study by Woo et al. in 2014 on 37 diabetic kidney disease patients and 40 healthy people showed that the concentration of cystatin C was 0.8 ± 0.2 mg/L in the healthy group. The CKD group had a cystatin C concentration of 4.2 ± 2.3 mg/L (11). The differences in the cystatin C concentrations in the CKD groups of all studies might be due to many factors such as the sample population size, characteristics of the age, gender, ethnicity, and treatment of the patients.
The limitation of this study was that the subject population size was small. The second limitation was that the study lacked CKD patients at the early stages, which caused a lack of information. Moreover, the concentration of cystatin C was not enough to estimate the changes throughout the disease development from the early stage to the end stage. Cystatin C level has shown clinical usefulness in CKD. This marker should be estimated in another follow-up study to get further results for clinical application.