Abstract
Background:
Detecting significant renal injury in an accurate and timely manner in acute kidney injury (AKI) patients who are critically ill remains controversial. Serum creatinine (Cr) is an important marker of kidney function in clinical practice, and its limitations are well known.Objectives:
This study aimed to evaluate neutrophil gelatinase-associated lipocalin (NGAL) as a marker of the early development of AKI in critically ill AKI patients.Methods:
This prospective study was carried out at JSS Hospital, Mysuru, India. The diagnosis and staging of AKI was done according to the RIFLE criteria.Results:
A total of 53 critically ill patients were enrolled in this study. During Intensive Care Unit (ICU) stay, 34 (64.2%) patients developed AKI according to RIFLE criteria. Serum NGAL levels assessed on admission were an appropriate predictor of AKI com-pared to serum Cr. Serum NGAL levels also showed a significant elevation among AKI patients than non-AKI cases. The mean levels for AKI patients at 0, 4, and 8 hours were 870.53, 1074.9, and 1090, respectively. Meanwhile, the mean levels for non-AKI patients at 0, 4, and 8 hours were 337, 307, and 292.Conclusions:
Measuring serum NGAL on admission is useful in the early diagnosis of AKI com-pared to serum Cr.Keywords
1. Background
Acute kidney injury (AKI) is a major healthcare problem in modern medicine. Ac-cording to the studies, 1% - 32% of the hospital admissions and 10% - 90% of intensive care unit (ICU) admissions are due to AKI (1, 2). This wide variation in prevalence of AKI is due to the different diagnostic criteria for defining AKI. In clinical practice, serum creatinine (Cr) is an important marker of kidney function. However, its diagnostic utility in AKI is limited by the delay from the onset of injury to the subsequent elevation in serum Cr.
Due to the restrictions of serum Cr to diagnose AKI, some new biomarkers such as neutrophil gelatinase-associated lipocalin (NGAL) have emerged as an appropriate biomarker of AKI. NGAL can be detected early after kidney damage in urine or plasma, and its concentration increases quickly, which represents the severity of the injury. This biomarker is noninvasive and can be accurately measured using standard methods.
2. Objectives
In this study, we evaluated the efficacy of serum NGAL as an indicator of the early development of AKI in critically ill AKI patients.
3. Methods
The current prospective research evaluated 53 patients admitted to the ICU of JSS hospital, Mysuru, India, from December 2013 to August 2015. Diagnosis and staging of AKI was done according to the RIFLE criteria.
3.1. Inclusion Criteria
The inclusion criteria were: patients aged over 18 years; hemodynamic instability or requiring vasopressors to maintain Blood pressure BP; multi-organ dysfunction including dysfunction of more than one organ, requiring intervention to maintain homeostasis; and sepsis.
3.2. Exclusion Criteria
The exclusion criteria were: cases with estimated glomerular filtration rate (CKD-EPI) < 60 ml/min; patients with a transplanted kidney; and unwillingness to participate in the study.
The subjects were assessed for the cause of AKI according to their history and clinical and laboratory data. Laboratory data, including serum Cr were assessed once a day as part of routine health care from the admission day. Urine output was routinely recorded at 6-hour intervals. Blood sampling for NGAL was done on admission and subsequently at 4 and 8 hours.
3.3. Description of the Test
The Triage® NGAL Test (Biosite Inc, CA) was applied to measure Plasma NGAL (pNGAL). This test is a rapid point-of-care and fluorescence-based immunoassay to assess NGAL level in whole blood/plasma samples with a lower limit of detection of 60 ng/mL. The pNGAL inter- and intra-assay coefficients of variation were < 15%, and pNGAL in 50 blood specimens (median three specimens per case) were measured. Serum Cr was measured using Jaffe’s reaction.
3.4. Statistical Analysis
The two-sample t-test or Wilcoxon rank-sum test was employed for comparing continuous variables, and the chi-square test or Fisher’s exact test was employed for dichotomous variables. The area under curve (AUC) was used for determining the ability of the biomarkers for discriminating between AKI and non-AKI cases. A receiver operating characteristic (ROC) was employed to determine whether the separation of the biomarker values between AKI and non-AKI cases differed by chronic kidney disease (CKD).
4. Results
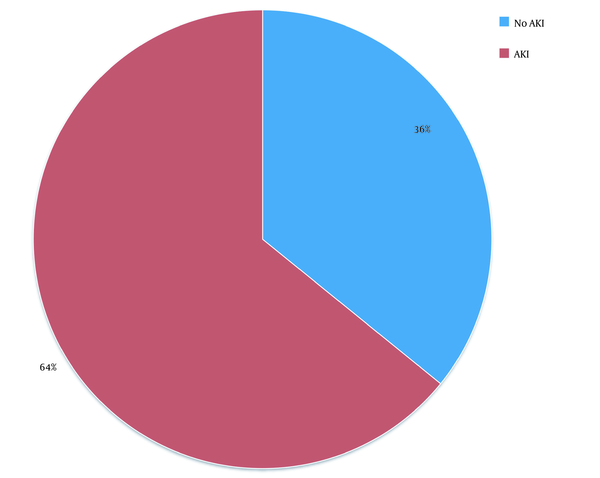
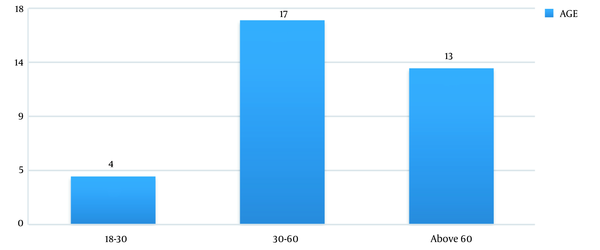
In this study, out of 100 cases admitted to the ICU from December 2013 to August 2015, 37 patients were excluded due to the underlying CKD and 10 cases due to post-renal transplant status. Finally, out of 53 patients (61.76% males vs. 38.24% females) enrolled in the study, 34 (64.2%) patients developed AKI according to the RI-FLE criteria (Figure 1). Table 1 presents patients’ clinical characteristics at baseline. The age range of our study population was 20 - 75 years, with the mean age being 55.6 ± 15.8 years (Figure 2). Table 2 depicts the levels of serum Cr at 0, 24, 48, and 72 hours after admission. The rise in serum Cr from baseline to 24 hours meets the AKI definition by RIFLE criteria. Serum Cr at baseline was 1.16 mg/dL, which increased to 1.96 at 24 hours, elevated to 2.61 at 48 hours, and remained almost the same (2.58) at 72 hours. Comparing the above values with the t-test, the rise in Cr at 24, 48, and 72 hours, respectively, was statistically significant (P < 0.001).
Distribution of AKI patients
Distribution of age among AKI patients
Baseline Demographics
| Variables | AKI Group | Non-AKI Group |
|---|---|---|
| Total patients | 34 | 19 |
| Mean age | 55.6 ± 15.8 | 50.3 ± 15.6 |
| Gender | ||
| Male | 21 | 10 |
| Female | 13 | 9 |
| Etiology for AKI | ||
| Sepsis | 27 | 7 |
| Other etiology | 7 | 12 |
| S. creatinine (mg/dL) mean (baseline) | 1.15 ± .18 | 0.97 ± 0.22 |
Serum Creatinine Level at Different Time Points with AKI
| AKI, Mean ± SD | |
|---|---|
| SC-ON-ADMISSION | 1.16 ± 0.18 |
| SC-24 h | 1.96 ± 0.40 |
| SC-48 h | 2.61 ± 0.99 |
| SC-72 h | 2.58 ± 1.17 |
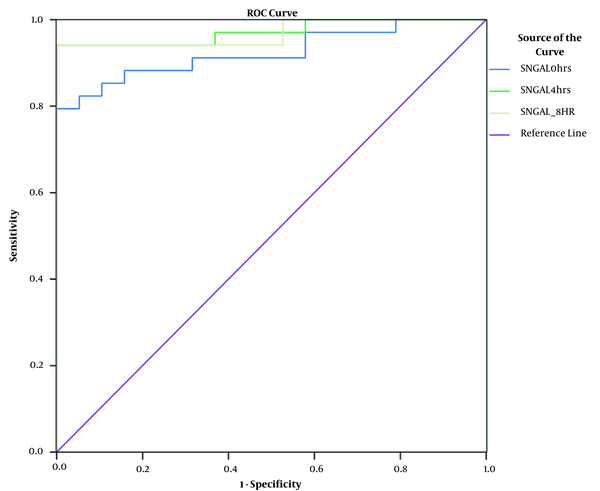
Serum NGAL concentrations were measured on admission, and subsequently after 4 and 8 hours. A significant increase in serum NGAL levels was observed at all three intervals. The AUC-ROC curve confirms the significantly elevated levels of serum NGAL at different time points (i.e. 0, 4, and 8 hours) in AKI patients (and and Table 3).
ROC curve showing AUC for predicting AKI using serum NGAL at different time points
The AUC for Determining AKI Using Serum NGAL at Different Time Intervals of ICU Stay
| Test Result Variable(s) | Area Under the Curve | ||
|---|---|---|---|
| Area | SE | P | |
| SNGAL 0 h | 0.924 | 0.036 | < 0.0001 |
| SNGAL 4 h | 0.972 | 0.021 | < 0.0001 |
| SNGAL 8 h | 0.969 | 0.023 | < 0.0001 |
5. Discussion
AKI is a common condition affecting critically ill patients with an incidence rate of 16% - 67% in the ICU setting. In a 10-year cohort research on over 90,000 patients from more than 20 ICUs, the incidence of AKI increased by 2.8% per year (3).
Serum Cr has been used as a common marker of AKI. However, it is an inadequate marker for AKI because there must be a substantial loss of glomerular filtration rate (GFR) before serum Cr level begins to rise, and that it cannot precisely indicate kidney function before steady state is achieved, which can take several days. Any intervention done once the Cr levels are elevated may be too late to improve the outcome. Hence, there is a need to identify a biomarker that can detect AKI in an early manner and prevent its further progression, thereby preventing mortality and morbidity. NGAL satisfies several characteristics crucial for an appropriate AKI biomarker. After renal injury, NGAL can be quickly expressed in the apical epithelial membranes of the distal nephron (4, 5), and its concentration increases by 10- to 100-fold within 2 hours of tubular injury. In this study, we evaluated the serum NGAL effects as an indicator of the early development of AKI in critically ill AKI cases.
In the current study, serum NGAL evaluated on admission was an appropriate predictor of AKI. AKI patients had significantly increased serum NGAL concentrations. The mean NGAL levels of AKI patients at 0, 4, and 8 hours were 870.53, 1074.9, and 1090, respectively. Accordingly, our results indicated that monitoring serum NGAL levels can provide the earliest warning for the development of AKI compared to serum Cr. This is consistent with the studies conducted by Mishra et al. (6) and Wheeler et al. (7), in which the serum NGAL levels during 24 hours of pediatric ICU admission showed a significant increase in children with AKI than those without AKI.
Most of the AKI biomarker studies use sensitivity, specificity, and predictive values for generating summary characteristics, more importantly the AUC-ROC curve (8). In our study, the AUC for serum NGAL at 4 hours was 0.97 with a sensitivity of 93% and specificity of 83%. Also, the AUC at 8 hours was 0.92 with a sensitivity of 78% and specificity of 80%. This is consistent with the research by Makris et al. (9) reporting that urinary NGAL concentrations assessed during 24 hours from injury is an appropriate early AKI marker in critically ill trauma cases characterized by AUC-ROC of 0.97 (10-12). Another investigation on children admitted to the ICU with sepsis, septic shock, and critically ill adults showed the serum and urinary NGAL accuracy in predicting AKI with AUCs of 0.68 and 0.64 for sustained AKI. Constantin et al. (13) and Nickolas et al. (14) reported very high AUCs for the ability of serum NGAL and urinary NGAL for predicting AKI in critically ill adults and emergency department patients (0.92 and 0.95, respectively). There are few studies on serum NGAL among adult ICU patients as an early marker of AKI, and the majority of studies have been carried out in pediatric setting.
Our research adds to the existing literature since we found that NGAL could significantly improve the diagnostic accuracy for early AKI even when patients had normal serum Cr levels at admission.
This study had some limitations. First, the sample size was small. Second, we did not compare the results with those of non-AKI patients. Third, although NGAL levels evaluated in critically ill patients during the first 24 hours of ICU admission, this may not definitely be the first 24 hours of the patients’ disease process.
5.1. Conclusions
It could be concluded that serum NGAL assessment is an effective marker for the ear-ly diagnosis of AKI. Baseline NGAL assessment can detect AKI earlier than serum Cr, which may allow for earlier and more favorable interventions.
References
-
1.
Bihorac A, Yavas S, Subbiah S, Hobson CE, Schold JD, Gabrielli A, et al. Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. Ann Surg. 2009;249(5):851-8. [PubMed ID: 19387314]. https://doi.org/10.1097/SLA.0b013e3181a40a0b.
-
2.
Kheterpal S, Tremper KK, Heung M, Rosenberg AL, Englesbe M, Shanks AM, et al. Development and validation of an acute kidney injury risk index for patients undergoing general surgery: results from a national data set. Anesthesiology. 2009;110(3):505-15. [PubMed ID: 19212261]. https://doi.org/10.1097/ALN.0b013e3181979440.
-
3.
Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34(2):344-53. [PubMed ID: 16424713]. https://doi.org/10.1097/01.ccm.0000194725.48928.3a.
-
4.
Yang J, Goetz D, Li JY, Wang W, Mori K, Setlik D, et al. An iron delivery pathway mediated by a lipocalin. Mol Cell. 2002;10(5):1045-56. [PubMed ID: 12453413]. https://doi.org/10.1016/s1097-2765(02)00710-4.
-
5.
Gwira JA, Wei F, Ishibe S, Ueland JM, Barasch J, Cantley LG. Expression of neutrophil gelatinase-associated lipocalin regulates epithelial morphogenesis in vitro. J Biol Chem. 2005;280(9):7875-82. [PubMed ID: 15637066]. https://doi.org/10.1074/jbc.M413192200.
-
6.
Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365(9466):1231-8. [PubMed ID: 15811456]. https://doi.org/10.1016/S0140-6736(05)74811-X.
-
7.
Wheeler DS, Devarajan P, Ma Q, Harmon K, Monaco M, Cvijanovich N, et al. Serum neutrophil gelatinase-associated lipocalin (NGAL) as a marker of acute kidney injury in critically ill children with septic shock. Crit Care Med. 2008;36(4):1297-303. [PubMed ID: 18379258]. [PubMed Central ID: PMC2757115]. https://doi.org/10.1097/CCM.0b013e318169245a.
-
8.
Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143(1):29-36. [PubMed ID: 7063747]. https://doi.org/10.1148/radiology.143.1.7063747.
-
9.
Makris K, Markou N, Evodia E, Dimopoulou E, Drakopoulos I, Ntetsika K, et al. Urinary neutrophil gelatinase-associated lipocalin (NGAL) as an early marker of acute kidney injury in critically ill multiple trauma patients. Clin Chem Lab Med. 2009;47(1):79-82. [PubMed ID: 19055468]. https://doi.org/10.1515/CCLM.2009.004.
-
10.
Schmidt-Ott KM, Mori K, Kalandadze A, Li JY, Paragas N, Nicholas T, et al. Neutrophil gelatinase-associated lipocalin-mediated iron traffic in kidney epithelia. Curr Opin Nephrol Hypertens. 2006;15(4):442-9. [PubMed ID: 16775460]. https://doi.org/10.1097/01.mnh.0000232886.81142.58.
-
11.
Schmidt-Ott KM, Mori K, Li JY, Kalandadze A, Cohen DJ, Devarajan P, et al. Dual action of neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol. 2007;18(2):407-13. [PubMed ID: 17229907]. https://doi.org/10.1681/ASN.2006080882.
-
12.
Axelsson L, Bergenfeldt M, Ohlsson K. Studies of the release and turnover of a human neutrophil lipocalin. Scand J Clin Lab Invest. 1995;55(7):577-88. [PubMed ID: 8633182]. https://doi.org/10.3109/00365519509110257.
-
13.
Constantin JM, Futier E, Perbet S, Roszyk L, Lautrette A, Gillart T, et al. Plasma neutrophil gelatinase-associated lipocalin is an early marker of acute kidney injury in adult critically ill patients: a prospective study. J Crit Care. 2010;25(1):176 e1-6. [PubMed ID: 19781900]. https://doi.org/10.1016/j.jcrc.2009.05.010.
-
14.
Nickolas TL, O'Rourke MJ, Yang J, Sise ME, Canetta PA, Barasch N, et al. Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann Intern Med. 2008;148(11):810-9. [PubMed ID: 18519927]. [PubMed Central ID: PMC2909852]. https://doi.org/10.7326/0003-4819-148-11-200806030-00003.