Abstract
Background:
Olfactory neuroblastoma or esthesioneuroblastoma is a rare malignant neoplasm of the olfactory neuroepithelium.Objectives:
This study aimed to investigate the clinical presentation, characteristics and treatment outcome of fourteen patients with olfactory neuroblastoma.Patients and Methods:
This retrospective study reviewed and analyzed 14 patients with olfactory neuroblastoma during the last 15 years at two referral tertiary academic hospitals of Shiraz University of Medical Sciences. Patients under 16 years old were excluded from the study. Kadish classification was used for staging the tumors. In addition, the tumors were histopathologically graded according to Hyams’ grading classification.Results:
There were 6 women (43%) and 8 men (57%) ranging from 16 to 56 years old, with a median age of 40 years at diagnosis. Nasal obstruction (93%), facial or sinus pain (57%), and nasal bleeding (43%) were the most frequent presentation. Two patients (14%) had stage A, 3 (21.5%) had stage B, 6 (43%) had stage C, and 3 (21.5%) had stage D disease. After initial treatment, five patients developed local and/or distant recurrent disease. After a median follow-up of 73 (range 14 - 108) months for surviving patients, 10 patients (71.5%) were alive and without disease, one (7%) was alive with disease, and 3 (21.5%) died due to the disease. The 5-year disease-free and overall survival rates were 71.4% and 75.7%, respectively.Conclusions:
At our center, patients with olfactory neuroblastoma tend to present at advanced stages; therefore, combined local treatment and incorporation of chemotherapy may improve outcome.Keywords
Olfactory Neuroblastoma Esthesioneuroblastoma Surgery Radiotherapy Chemotherapy
1. Background
Sinonasal tract cancers are uncommon and account for less than 1% of all malignancies. These neoplasms are a broad range of pathologies that tend to present at a locally advanced stage, have a high rate of local recurrence, and a moderate to poor outcome. The majority (approximately two thirds) of these malignancies are epithelial tumors (1). Squamous cell carcinoma, minor salivary gland tumors, and undifferentiated carcinoma are the most common epithelial malignancies in this location. Lymphomas, malignant melanoma, soft tissue sarcoma and olfactory neuroblastoma are the most common nonepithelial pathologies (1-3). Olfactory neuroblastoma or esthesioneuroblastoma is a rare malignant neuroectodermal neoplasm of the olfactory neuroepithelium. This tumor accounts for 3% - 8% of all sinonasal neoplasms and shows a broad biological behavior and natural history from indolent growth pattern with long-term survival to highly aggressive tumor with rapid disseminated disease and ominous outcome (1, 4-6). Histopathologically, olfactory neuroblastoma consisted of round, oval, or fusiform cells containing neurofibrils with pseudorosette formation with diffusely increased microvascularity (7). Accordingly, this tumor should be differentiated from a group of aggressive malignant tumors composed of monotonous undifferentiated small and blue-staining cells such as lymphomas, malignant melanoma, undifferentiated sinonasal carcinoma, Ewing’s sarcoma, and sinonasal neuroendocrine carcinoma. Immunohistochemical studies play a major role in pathologic diagnosis and differentiation from the other pathologies (7, 8). Nasal obstruction and epistaxis are the most frequent clinical presentation in patients with olfactory neuroblastoma (4, 9).
2. Objectives
This study aimed to investigate the clinical presentation, characteristics and treatment outcome of fourteen patients with olfactory neuroblastoma.
3. Patients and Methods
This retrospective study was carried out at two referral tertiary academic hospitals of Shiraz University of Medical Sciences. The characteristics, treatment outcome, and survival of all patients with histologically-proven olfactory neuroblastoma that were treated and followed-up between January 2000 and December 2014 were evaluated and analyzed. Patients under 16 years old were excluded from the study. Kadish classification was used for staging tumors. According to this staging classification, tumors that are confined to the nasal cavity are considered as Kadish stage A. Tumors that are confined to the nasal cavity and paranasal sinuses are classified as Kadish stage B; whereas Kadish stage C includes tumors beyond the nasal cavity and paranasal sinuses, including involvement of the cribriform plate, base of the skull, orbit or intracranial cavity. Finally, Kadish stage IV, are tumors with metastasis to cervical lymph nodes or distant metastasis. In addition, the tumors were histopathologically graded according to Hyams’ grading classification. Preliminary evaluation included a comprehensive medical history, clinical examination including panendoscopy (oro-haryngolaryngoscopy and rhinonasopharyngoscopy), chest radiography, CT scan and/or MRI of the head and neck region. Additional studies including chest, abdominal and pelvic CT scan and whole body bone scintigraphy that were performed for selected patients.
Immunohistochemical staining consisted of a panel of antigens of S-100, cytokeratin, desmin, vimentin, actin, glial fibrillary acidic protein, UMB45, and common leucocytic antigen (CD45) that was used to confirm the diagnosis and exclude other differential pathologies. Surgical treatment included open or endoscopic biopsy in three patients (21%), partial endoscopic resection in 7 patients (50%) and complete surgical resection (as maxillectomy and/or ethmoidectomy and/or turbinectomy) in 4 patients (29%). No craniofacial resection was performed for the patients. External beam radiotherapy using a 6 - 9 MV linear accelerator was carried out for all but one patient and a median total dose of 58 (range 30 - 60) Gy was delivered. Radiotherapy was delivered with either two-dimensional conventional or three-dimensional conformal radiotherapy. All radiation treatments were delivered using conventional fractionation as daily fraction of 1.8 - 2 Gy and five fractions per week. Five patients received neoadjuvant or adjuvant chemotherapy. In addition, salvage chemotherapy was used in five patients with relapsed or persistent disease. A median 4 (range 2 - 6) cycles of various chemotherapy combinations including etoposide and cisplatin; or etoposide and carboplatin; or vincristine, doxorubicin and cyclophosphamide were administered.
The date of surgery or biopsy was considered as the time of diagnosis. Survival durations were calculated from the date of diagnosis until the events of tumor regrowth, death due to any cause or the last follow-up. Disease-free survival was defined from the date of diagnosis to the date of disease recurrence at any site. Overall survival was defined from the date of diagnosis to date of death due to any cause. Clinical and pathological variables were analyzed using IBM SPSS, version 19 statistical software. Disease-free survival and overall survival rates were calculated using the Kaplan-Meier method.
4. Results
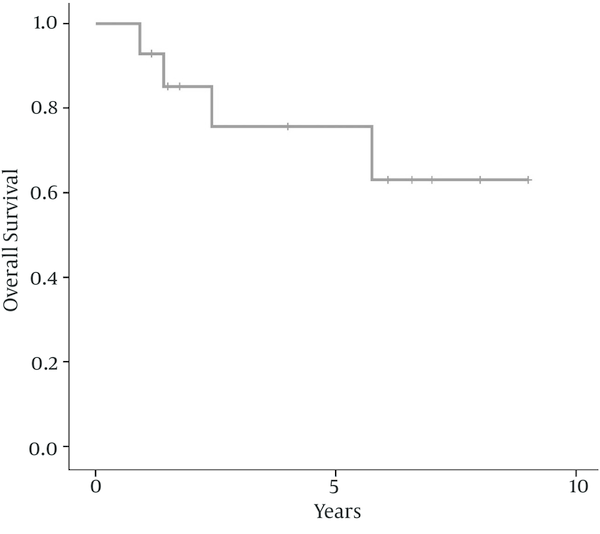
There were six women (43%) and 8 men (57%) ranging from 16 to 56 years old, with a median age of 40 years at diagnosis. The peak incidence was during the fifth decade of life in both genders. Nasal obstruction (93%), facial or sinus pain (57%), and nasal bleeding (43%) were the most frequent presentation followed by headache (43%), and hearing loss (14%). Two patients (14%) had stage A, 3 (21.5%) had stage B, 6 (43%) had stage C and 3 (21.5%) had stage D disease. The median largest tumor diameter was 5.5 (range 3 - 9) cm. According to Hyams’ grading classification, five patients (36%) had grade 1, 3 (21.5%) had grade 2, 4 (28.5%) had grade 3 and 2 (14%) had grade 4. Table 1 represents the details of clinical and pathological characteristics, treatment modality, and outcome of 14 patients with olfactory neuroblastoma. After initial treatment, five patients developed recurrent disease. One patient (7%) developed local recurrence, one (7%) developed distant failure, and two (14%) patients developed local and distant failure. Salvage chemotherapy alone with or without radiotherapy was used for patients with persistent or recurrent disease. After a median follow-up of 73 (range 14 - 108) months for surviving patients, 10 patients (71.5%) were alive and without disease, one (7%) were alive with disease, and 3 (21.5%) died due to the disease. The 5-year and 10-year disease free survival rates were 71.4% and 59.5%, respectively; as shown in Figure 1. The 5- and 10-year overall survival rates were 75.7% and 63.1%, respectively; as shown in Figure 2. In this study, because of the small sample size and few numbers of the events, we did not find a prognostic factor in univariate or multivariate analysis.
Details of Characteristics, Treatment Modality, and Outcome of 14 Patients With Olfactory Neuroblastoma
| Case | Sex | Age, y | Tumor Size | Hyams’ Grade | Kadish Stage | Initial Treatment | RT Dose (Gy) | Relapse | Salvage Treatment | Final Status |
|---|---|---|---|---|---|---|---|---|---|---|
| F | 45 | 6.0 | 1 | C | S + RT | 58 | Yes (62) | ChT | DOD (69) | |
| M | 26 | 4.0 | 1 | B | S + RT | 58 | No (96) | - | NED (96) | |
| M | 42 | 6.0 | 3 | D | S + ChT | - | Yes (7) | ChT+ RT | AWD (21) | |
| M | 56 | 5.0 | 2 | C | S + RT | 58 | No (73) | - | NED (73) | |
| F | 42 | 3.0 | 1 | A | ChT + RT | 60 | No (48) | - | NED (48) | |
| M | 38 | 5.0 | 2 | B | S + RT | 59 | No (79) | - | NED (79) | |
| F | 21 | 5.0 | 1 | B | S + RT | 56 | No (84) | - | NED (84) | |
| F | 21 | 9.0 | 3 | C | S + RT | 50 | Yes (11) | ChT | DOD (17) | |
| M | 22 | 7.0 | 3 | D | S + ChT + RT | 30 | Yes (7) | ChT | DOD (29) | |
| F | 16 | 6.5 | 4 | C | S + RT | 60 | Yes (4) | ChT | DOD (11) | |
| M | 16 | 4.0 | 3 | C | S + RT | 54 | No (108) | - | NED (108) | |
| M | 48 | 9.0 | 1 | C | ChT + RT | 60 | No (18) | - | NED (18) | |
| M | 48 | 6.0 | 2 | D | ChT + RT | 60 | No (14) | - | NED (14) | |
| F | 40 | 3.0 | 4 | A | S + RT | 60 | No (48) | - | NED (48) |
Kaplan-Meier Survival Curves of Disease-Free Survival in 14 Patients With Olfactory Neuroblastoma

Kaplan-Meier Survival Curves of Overall Survival in 14 Patients With Olfactory Neuroblastoma
5. Discussion
Olfactory neuroblastoma is a rare tumor of the sinonasal tract that affects all age groups and without significant sex predilection (4). In the current series, by excluding patients under 16 years old, the median age was 40 years at diagnosis with a slight male predominance. The majority of our patients presented at an advanced Kadish stage and were treated with multimodality treatment including surgery, radiotherapy and chemotherapy. This finding is consistent with the results of other studies that showed delayed diagnosis, usually due to the vague and nonspecific initial symptoms of this disease (10-12).
There is as yet, no consensus regarding the optimal treatment for olfactory neuroblastoma (13). However, according to several individual series, a multimodality approach particularly in advanced stage is preferred (14, 15). Many investigators believe that surgery is the mainstay of treatment and adjuvant radiotherapy will enhance local control and overall survival (16, 17). Seventy percent of our patients underwent surgery and then received adjuvant radiotherapy. Review of the literature demonstrated that surgery followed by postoperative radiotherapy is the most frequently used treatment (18, 19).
The role of adjuvant or salvage chemotherapy, either concurrent or sequential, with single or combination regimen and preferred drugs is another area of controversy. Mclean et al. found that there is no any survival benefit when cisplatin plus etoposide was added to the surgery in patients with olfactory neuroblastoma (20). Conversely, there are retrospective studies that suggested the potential benefit of adjuvant chemotherapy in high grade and advanced stage disease (21, 22). We used neoadjuvant and/or adjuvant chemotherapy in five of 14 patients, mainly combined with radiation and in advanced Kadish stage.
Locoregional recurrence and distant metastasis are the most common cause of treatment failure, and can occur several years later. The rate of local recurrence and distant metastasis was reported between 26% to 62% and 12% to 25%, respectively (4, 23). Radiotherapy, surgery and chemotherapy either alone or in combination has been used in different studies as salvage treatment depending on the clinical situation and the primary treatment (24, 25). In our study, five (35%) patients developed local and/or distant metastasis. All of them had advanced stage and high grade tumors. This finding is consistent with patient series that reported Kadish stage and Hyams’ grading as two important prognostic indicators in olfactory neuroblastoma (5, 26, 27). In our recurrent cases, surgery followed by adjuvant radiotherapy was the primary treatment of four patients, who were salvaged with chemotherapy. One patient who did not receive adjuvant radiotherapy was salvaged with chemoradiation. Salvage treatment in our patients was not successful. Four patients died and one was alive with disease in the last follow up. It seems that other modalities particularly re-irradiation with new modern techniques can be used in this setting (28).
In our study, the 5-year disease free and overall survival was 71% and 75%, respectively. In a study of Tajudeen et al. the 5-year disease free and overall survival was 54% and 82%, respectively (9). Most of the individual series reported disease free survival around 70% (25, 28-30). A report from Petruzzelli et al. showed disease free survival of 84% in retrospective analysis of 37 patients. They received multimodality treatment including surgery and chemoradiation. In that study, nine patients received neoadjuvant chemoradiation and half of them were treated with IMRT, which may explain the high disease-free survival (19). The best primary and salvage approach for patients with olfactory neuroblastoma should be investigated in future prospective studies.
At our center, patients with olfactory neuroblastoma tend to present at advanced stages; therefore, combined local treatments and incorporation of chemotherapy may improve outcome.
References
-
1.
Khademi B, Moradi A, Hoseini S, Mohammadianpanah M. Malignant neoplasms of the sinonasal tract: report of 71 patients and literature review and analysis. Oral Maxillofac Surg. 2009;13(4):191-9. [PubMed ID: 19795137]. https://doi.org/10.1007/s10006-009-0170-8.
-
2.
Khademi B, Bahranifard H, Nasrollahi H, Mohammadianpanah M. [Primary mucosal melanoma of the sinonasal tract: report of 18 patients and analysis of 1077 patients in the literature]. Braz J Otorhinolaryngol. 2011;77(1):58-64. [PubMed ID: 21340190].
-
3.
Khademi B, Owji SM, Khosh KJ, Mohammadianpanah M, Gandomi B. Description of a neural sheath tumor of the trigeminal nerve: immunohistochemical and electron microscopy study. Sao Paulo Med J. 2006;124(6):333-5. [PubMed ID: 17322954].
-
4.
Dulguerov P, Allal AS, Calcaterra TC. Esthesioneuroblastoma: a meta-analysis and review. Lancet Oncol. 2001;2(11):683-90.
-
5.
Jethanamest D, Morris LG, Sikora AG, Kutler DI. Esthesioneuroblastoma: a population-based analysis of survival and prognostic factors. Arch Otolaryngol Head Neck Surg. 2007;133(3):276-80. [PubMed ID: 17372086]. https://doi.org/10.1001/archotol.133.3.276.
-
6.
Bak M, Wein RO. Esthesioneuroblastoma: a contemporary review of diagnosis and management. Hematol Oncol Clin North Am. 2012;26(6):1185-207. [PubMed ID: 23116576]. https://doi.org/10.1016/j.hoc.2012.08.005.
-
7.
Thompson LD. Olfactory neuroblastoma. Head Neck Pathol. 2009;3(3):252-9. [PubMed ID: 20596981]. https://doi.org/10.1007/s12105-009-0125-2.
-
8.
Faragalla H, Weinreb I. Olfactory neuroblastoma: a review and update. Adv Anat Pathol. 2009;16(5):322-31. [PubMed ID: 19700942]. https://doi.org/10.1097/PAP.0b013e3181b544cf.
-
9.
Tajudeen BA, Arshi A, Suh JD, Palma-Diaz MF, Bergsneider M, Abemayor E, et al. Esthesioneuroblastoma: an update on the UCLA experience, 2002-2013. J Neurol Surg B Skull Base. 2015;76(1):43-9. [PubMed ID: 25685649]. https://doi.org/10.1055/s-0034-1390011.
-
10.
Oskouian RJJ, Jane JAS, Dumont AS, Sheehan JM, Laurent JJ, Levine PA. Esthesioneuroblastoma: clinical presentation, radiological, and pathological features, treatment, review of the literature, and the University of Virginia experience. Neurosurg Focus. 2002;12(5). e4. [PubMed ID: 16119902].
-
11.
Theilgaard SA, Buchwald C, Ingeholm P, Kornum Larsen S, Eriksen JG, Sand Hansen H. Esthesioneuroblastoma: a Danish demographic study of 40 patients registered between 1978 and 2000. Acta Otolaryngol. 2003;123(3):433-9. [PubMed ID: 12737303].
-
12.
Tsang RK, Tsang AC, Lam JW, Ho WK, Leung GK. Long-term results of endoscopic-assisted cranionasal resection for olfactory neuroblastoma - single centre experience of fourteen patients. Clin Otolaryngol. 2015;40(3):274-7. [PubMed ID: 25522034]. https://doi.org/10.1111/coa.12364.
-
13.
Proust F. Treatment of esthesioneuroblastoma: a double approach? Neurochirurgie. 2015;61(1):54. [PubMed ID: 25595593]. https://doi.org/10.1016/j.neuchi.2014.09.004.
-
14.
Jarzabski A, Kiprian D, Szutkowski Z, Kawecki A, Michalski W. [The results of combined treatment patients with olfactory neuroblastoma in material of Cancer Center in Warsaw]. Otolaryngol Pol. 2011;65(5 Suppl):93-7. [PubMed ID: 22000257]. https://doi.org/10.1016/S0030-6657(11)70715-6.
-
15.
Modesto A, Blanchard P, Tao YG, Rives M, Janot F, Serrano E, et al. Multimodal treatment and long-term outcome of patients with esthesioneuroblastoma. Oral Oncol. 2013;49(8):830-4. [PubMed ID: 23747132]. https://doi.org/10.1016/j.oraloncology.2013.04.013.
-
16.
Diaz EMJ, Johnigan RH, Pero C, El-Naggar AK, Roberts DB, Barker JL, et al. Olfactory neuroblastoma: the 22-year experience at one comprehensive cancer center. Head Neck. 2005;27(2):138-49. [PubMed ID: 15654688]. https://doi.org/10.1002/hed.20127.
-
17.
Platek ME, Merzianu M, Mashtare TL, Popat SR, Rigual NR, Warren GW, et al. Improved survival following surgery and radiation therapy for olfactory neuroblastoma: analysis of the SEER database. Radiat Oncol. 2011;6:41. [PubMed ID: 21518449]. https://doi.org/10.1186/1748-717X-6-41.
-
18.
Chao KS, Kaplan C, Simpson JR, Haughey B, Spector GJ, Sessions DG, et al. Esthesioneuroblastoma: the impact of treatment modality. Head Neck. 2001;23(9):749-57. [PubMed ID: 11505485].
-
19.
Petruzzelli GJ, Howell JB, Pederson A, Origitano TC, Byrne RW, Munoz L, et al. Multidisciplinary treatment of olfactory neuroblastoma: Patterns of failure and management of recurrence. Am J Otolaryngol. 2015;36(4):547-53. [PubMed ID: 25749259]. https://doi.org/10.1016/j.amjoto.2015.02.008.
-
20.
McLean JN, Nunley SR, Klass C, Moore C, Muller S, Johnstone PA. Combined modality therapy of esthesioneuroblastoma. Otolaryngol Head Neck Surg. 2007;136(6):998-1002. [PubMed ID: 17547995]. https://doi.org/10.1016/j.otohns.2006.11.051.
-
21.
McElroy EAJ, Buckner JC, Lewis JE. Chemotherapy for advanced esthesioneuroblastoma: the Mayo Clinic experience. Neurosurgery. 1998;42(5):1023-7. [PubMed ID: 9588546].
-
22.
Porter AB, Bernold DM, Giannini C, Foote RL, Link MJ, Olsen KD, et al. Retrospective review of adjuvant chemotherapy for esthesioneuroblastoma. J Neurooncol. 2008;90(2):201-4. [PubMed ID: 18633576]. https://doi.org/10.1007/s11060-008-9645-y.
-
23.
Resto VA, Eisele DW, Forastiere A, Zahurak M, Lee DJ, Westra WH. Esthesioneuroblastoma: the Johns Hopkins experience. Head Neck. 2000;22(6):550-8. [PubMed ID: 10941155].
-
24.
Kim HJ, Cho HJ, Kim KS, Lee HS, Kim HJ, Jung E, et al. Results of salvage therapy after failure of initial treatment for advanced olfactory neuroblastoma. J Craniomaxillofac Surg. 2008;36(1):47-52. [PubMed ID: 18312791]. https://doi.org/10.1016/j.jcms.2007.08.004.
-
25.
Song CM, Won TB, Lee CH, Kim DY, Rhee CS. Treatment modalities and outcomes of olfactory neuroblastoma. Laryngoscope. 2012;122(11):2389-95. [PubMed ID: 23070733]. https://doi.org/10.1002/lary.23641.
-
26.
Tajudeen BA, Arshi A, Suh JD, St John M, Wang MB. Importance of tumor grade in esthesioneuroblastoma survival: a population-based analysis. JAMA Otolaryngol Head Neck Surg. 2014;140(12):1124-9. [PubMed ID: 25356502]. https://doi.org/10.1001/jamaoto.2014.2541.
-
27.
Bell D, Saade R, Roberts D, Ow TJ, Kupferman M, DeMonte F, et al. Prognostic utility of Hyams histological grading and Kadish-Morita staging systems for esthesioneuroblastoma outcomes. Head Neck Pathol. 2015;9(1):51-9. [PubMed ID: 24806334]. https://doi.org/10.1007/s12105-014-0547-3.
-
28.
Nishimura H, Ogino T, Kawashima M, Nihei K, Arahira S, Onozawa M, et al. Proton-beam therapy for olfactory neuroblastoma. Int J Radiat Oncol Biol Phys. 2007;68(3):758-62. [PubMed ID: 17398027]. https://doi.org/10.1016/j.ijrobp.2006.12.071.
-
29.
Rimmer J, Lund VJ, Beale T, Wei WI, Howard D. Olfactory neuroblastoma: a 35-year experience and suggested follow-up protocol. Laryngoscope. 2014;124(7):1542-9. [PubMed ID: 24347437]. https://doi.org/10.1002/lary.24562.
-
30.
De Bonnecaze G, Chaput B, Al Hawat A, Filleron T, Vairel B, Serrano E, et al. Long-term oncological outcome after endoscopic surgery for olfactory esthesioneuroblastoma. Acta Otolaryngol. 2014;134(12):1259-64. [PubMed ID: 25399885]. https://doi.org/10.3109/00016489.2014.944271.