Abstract
Keywords
1. Introduction
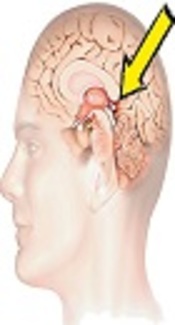
The pineal region is not a common tumor site and accounts for only 1% of the central nervous system (CNS) neoplasms. In pediatric patients, pineal tumors constitute 3-4% of CNS tumors. Pineal parenchymal tumors, meningioma, germ cell tumor, glioma metastasis, and arachnoid cyst are the main differential diagnoses in the pineal region (1).
For the first time, Jouvet et al. in 2003 reported six patients with a new disease of the papillary region. They introduced a new clinicopathological disease, known as the papillary tumor of pineal region (PTPR) (2). PTPR originates from ependymal cells in the subcommissural organ (SCO) (3). SCO appears early in embryonic life and is present in the subphylum vertebrata. It is among the first differentiated organs in the brain and develops in the second month of fetal life. Its activity decreases before birth and continues to regress throughout adulthood; however, its function is not well understood. Some molecules are also secreted from this organ to the cerebrospinal fluid (4, 5).
In 2007, the World Health Organization (WHO) recognized PTPR as a distinguished disease in children and adults (6). Since then, more cases have been reported in the literature. Considering the rarity of this tumor, all reported series are retrospective and there are no prospective studies. In this study, we collected all English - language reports on PTPR and attempted to clarify some aspects of this disease.
2. Methods
We searched PubMed for relevant articles. The main keyword was “papillary tumor of pineal region”. The full - texts of all articles were collected and reviewed, while articles without any accessible or available full - texts were excluded. Articles reporting case(s) from the archives or autopsy reports were also removed. Other exclusion criteria were review articles and animal studies. Afterwards, demographic characteristics, type of surgery, adjuvant treatment, disease, overall survival, and disease - free survival (DFS) rate were retrieved from the reports, and analyses were performed accordingly.
3. Results
In August 2016, 72 articles were retrieved from PubMed, while 19 articles were excluded. Data from 53 reports, including 73 patients, were collected. Nineteen articles were excluded, as they contained no clinical data (five studies) or were review articles (seven studies), which had found PTPR by reviewing the archive slides. Also, we had no access to the full - text of four articles; one article had reported PTPR in a canine, and two articles were not published in English.
The mean age of the patients was 33.5 (± 15.4) years. The youngest patient was 15 months old, while the oldest patient was 70 years old. Overall, 40 patients were male and 31 were female. The majority of reports were from USA (21 cases), Europe (25 cases), Asia (18 cases), Brazil (2 cases), and Australia (3 cases). We only found one report from the Middle East (Saudi Arabia), while in three cases, the country was not mentioned.
The presentations were not mentioned in 11 patients. The most common symptom was headache (80%). In some cases, headache was observed along with other complaints, such as nausea (31%) and hydrocephalus or impaired gait (2%). Visual complaints were also common, as reported in 29 (40%) patients; in 22 cases, they were in conjunction with headache. The visual problems included diplopia, visual disturbance, photophobia, nystagmus, and blurred vision. Nausea and vomiting were observed in six patients. One patient had convulsion, while five patients had memory loss. Also, three cases had Parinaud’s syndrome, and two cases had vertigo; most patients had a combination of symptoms.
In total, 34 patients had undergone complete tumor removal. In 18 and 10 patients, partial tumor resection and biopsy alone were performed, respectively. Information about the type of surgery was not well defined in 11 patients. External beam radiotherapy (RT) was performed in 30 patients; one of the patients received craniospinal irradiation. Stereotactic radiosurgery (SRS) was applied for ten patients, while five patients received brachytherapy.
Eleven patients did not receive adjuvant RT, and information was incomplete in 19 reports. One case received 24 - Gy whole brain RT (WBRT) up to 54 Gy in the reduced field; another patient also received 30 - Gy WBRT. The dose of conventional RT was reported in only 19 patients, and the median RT dose was 50 Gy (45 - 59.4 Gy). Also, one patient received proton therapy.
Eleven patients had received chemotherapy as treatment after diagnosis. In three reports, the used agents were not mentioned. The chemotherapy agents and regimens included: cisplatin, etoposide, and ifosfamide (two cases); chloroethylnitrosourea (one case); etoposide, carboplatin, and temodal (one case); temodal and etoposide (one case); temodal (two cases); and carboplatin, etoposide, and vincristine (one case).
Fifty patients had no evidence of recurrence at the time of report. Six patients had meningeal seeding, and 21 patients had local recurrence. Three patients had local recurrence and parenchymal metastasis, while two patients showed both local recurrence and meningeal seeding. The parenchymal sites included the occipital lobe, frontal lobe, and cerebellum. It should be noted that only three patients had died at the time of report.
The median DFS was 24 months, while five- and ten - year DFS rates were 50.1% and 25.0%, respectively; the median overall survival was 24 months. No mortality was reported in 60 to 120 months, and the five- and ten - year overall survival rates were similar to DFS. In the univariate analysis, only RT (P = 0.037), chemotherapy (P = 0.001), and treatment type (P = 0.003) were prognostic factors for five - year DFS. Type of surgery (P = 0.531), sex (P = 0.072), age (P = 0.920), and RT dose (P = 0.327) were not significant prognostic factors for five - year DFS. Regarding the total number of patients and those receiving chemotherapy, further reports with longer follow - ups (F/U) are required in order to draw a definite conclusion.
4. Discussion
Presentations of PTPR can be attributed to its location, mass effect, and consequent increment in intracranial pressure (ICP) (7). In our review, the most common presentation was headache. Tumor presentation was not a significant factor for survival. The age distribution of PTPR patients was remarkable. The youngest patient was an infant (a 15 - month - old boy), while the oldest patient was 70 years old (1, 8). Three patients were younger than ten years (1.25, 3, and 4 years). In total, 10, 20, 11, 12, 12, 3, and 1 patient were in the second, third, fourth, fifth, sixth, seventh, and eighth decades of life, respectively. It seems that this tumor may occur throughout life in both genders, and age is not a significant factor for survival.
Treatment of PTPR is not well - defined, and no standard guidelines are available. A wide range of treatments has been proposed in the literature. On the other hand, we found several reports about patients who had not received any treatments for different reasons. One patient was followed - up for two years. No intervention had been applied for this patient when he became symptomatic (9).
PTPR may cause hydrocephalus, and ICP reduction is occasionally necessary. Some patients were well for a relatively long period and received no treatment, except for shunt insertion. Three patients with shunt insertions were in a good condition for 2.5, 3.5, and 4 years, respectively (10-12). PTPR can be risky for patients if left without treatment. In this regard, Cohan reported the case of a 29 - year - old man, who refused treatment, and as a result, his lesion increased in size within eight months. Even though the increment in size was small, his symptoms increased significantly (13). We also found other cases with worse clinical courses. In two separate reports, the patients were readmitted to hospital after six and four months, respectively, and one of them showed leptomeningeal seeding (14, 15). It seems that PTPR cannot be neglected, and close monitoring is essential under special circumstances.
Complete tumor resection in the pineal region is not simple. Up to 18% of severe side effects, such as Parinaud’s syndrome, hemiparesis, memory disturbances, and cranial nerve palsy, have been reported in pineal region surgeries (9). In our review, 32 (45%) patients had complete tumor resection. Some patients received no adjuvant treatments after total tumor resection. Epari reported the case of a 37 - year - old woman, who had no tumor recurrence in 2.5 years of follow - up (16). There are some reports of tumor control after surgery alone, which was found to be effective for eight and 15 months after surgery, respectively (17, 18). On the other hand, Sun presented a 23 - year - old woman with gross total (GT) resection, who experienced tumor recurrence and became symptomatic. Reoperation and RT were applied for this patient (19). It seems that adjuvant RT is beneficial following complete tumor removal. In our analysis, type of surgery was not a significant prognostic factor.
We found 18 patients with partial tumor resection. Gutenberg reported a patient, who remained disease - free for seven years. The patient’s tumor had not been completely removed, and she had received brachytherapy (20). On the other hand, we found two cases of PTPR with incomplete tumor resection. The patients received 50- and 55 - Gy radiation, as well as chemotherapy. Although local recurrence occurred twice, the patients were well and alive for 218 and 240 months after presentation, respectively (21-24). There are other reports of PTPR in patients who did not undergo complete tumor resection and remained well for a long period (84 and 108 months, respectively) (9, 25). It seems that local recurrence is the main problem after surgery; therefore, cerebrospinal fluid seeding is possible.
RT can be a good therapeutic option in PTPR. In a case report, a pineal lesion was found, and only biopsy was performed. The patient received 50.4 - Gy irradiation to the lesion, and after three months, the lesion completely disappeared (26). In general, RT can be administered before surgery. Nakamura reported the case of a patient who received RT before surgery. The patient was treated for a primitive neuroectodermal tumor, and accordingly, RT and chemotherapy were performed; the patient remained well for 15 years (27). However, there are several reports indicating poor response to RT. In this regard, Shibahara reported a 29 - year - old man who did not respond to first - line RT (50 Gy) and underwent surgery again after three months (3).
Several irradiation doses and techniques have been used in different reports. In our review, the median conventional RT dose was 50 Gy (45 - 59 Gy). Conventional RT was used in most reports (28 cases). The highest RT dose was reported by Cohen, who irradiated the patient up to 59.4 Gy in 33 fractions. The patient showed parenchymal recurrence seven months after therapy (23). In another report, the patient received 30 - Gy RT in ten fractions and remained alive for 56 months (28). In addition, Inoue reported a case of PTPR, who received 24 - Gy irradiation to the whole brain and 30 - Gy irradiation to the tumor (29). In seven cases, RT dose was not mentioned; overall, RT dose was not a prognostic factor for survival.
In nine reports, ten patients received SRS, which was used as an adjuvant or primary treatment (2, 8, 9, 25, 28, 30-33). In four reports, the patients had no recurrence for 23, 60, 84, and 108 months, respectively (2, 9, 25, 28). Moreover, Cardens reported the case of a 47 - year - old man, who received SRS without biopsy. His lesion decreased in size, but it reappeared after seven years (33). In another report, SRS was not quite successful, and the patient developed multiple local recurrences. The patient had a relatively prolonged survival and underwent SRS three times during 14 years (32). Although SRS is effective against PTPR, the response rate and response duration to SRS require more investigation.
Regarding proton beam RT, we found two articles, which applied proton beam RT to treat PTPR. Proton RT produced complete tumor responses in a four - year - old child (1). Another RT technique is brachytherapy. In a previous report, four cases of PTPR showed good response to brachytherapy. Iodine - 125 seeds were used as the source of radiation. One case showed complete response after 108 months, and in one case, the disease became stable after 13 months. Two patients showed partial response after 87 and 20 months of follow - up, respectively (34). In addition, Gutenberg used iodine - 125 seeds for a female patient; she was well for seven years, and the tumor size was stable (20). Accordingly, brachytherapy seems to be a good therapeutic option.
In 12 cases, chemotherapy was administered, which was a significant factor for survival. In a report, cisplatin and etoposide decreased PTPR close to half of its volume (21). Nonetheless, cisplatin, etoposide, and ifosfamide were not effective in leptomeningeal seeding (15). Shibahara administered adjuvant chemotherapy (ifosfamide, cisplatin, and etoposide) after RT in a 29 - year - old woman. The patient was well for nine months, but the follow - up was not long enough to draw a conclusion (3).
Lorenzetti administered adjuvant temozolomide and maintained it in a 42 - year - old patient for 26 cycles. The patient’s tumor progressed after RT, although it showed good response to temozolomide (35). In addition, Shakir reported the case of a 31 - year - old man with PTPR. After partial resection, he received temozolomide for one year; then, SRS was performed, and temozolomide was administered for another year. The tumor volume decreased from 4.2 cm3 to 3.5 cm3; the patient was well, and the tumor decreased to 1.3 cm3 after nine years (25).
In addition, Cohen reported the case of a 31 - year - old man with no response to teozolamide. He had recurrent PTPR after several sessions of RT. He received 11 courses of temozolomide (75 mg/m2) for 21 days, once every 4 weeks, with no response. On the other hand, bevacizumab, as a single agent, was partially effective against PTPR. The tumor decreased in size and became stable for 13 months (it increased again afterwards) (23).
Lechapt - Zalcman reported a case of PTPR with three recurrences over eight years. After the third recurrence, the patient received nine cycles of etoposide and carboplatin. He was well for six years, when recurrence occurred (22). At the time of report, 42 patients had no recurrence. The most common type of recurrence was local (23 patients); six patients had meningeal seeding, and three patients had parenchymal metastasis. In this regard, Sato, Hong, and Kim reported three cases with short survival after cerebrospinal fluid seeding, while Nowicka reported a case with an indolent course despite spinal dissemination (15, 30, 32, 36).
Some patients had a prolonged disease - free course (54, 56, 60, 84, 108, 119, and 180 months). GT resection was applied for four patients, and near - total (NT) resection was used for two cases. One patient underwent biopsy. The RT dose was 50.4 Gy in one patient and 54 Gy in two patients. SRS was applied in three patients. One case received 30 - Gy radiation in ten fractions, and only one patient received chemotherapy (9, 16, 25, 27, 28, 37).
We also found some reports on parenchymal metastasis. Lechapt - Zalcman reported a long follow - up in a 21 - year - old man with multiple recurrences in the cerebellum. Three years after treating the primary tumor, two new lesions appeared in the cerebellum. Metastatic lesions were removed, and RT to the posterior fossa was applied. He developed cerebellum and local recurrences six and eight years later and underwent surgery twice. A new lesion appeared in the cerebellum 14 years after the primary diagnosis, and he received temozolomide. He underwent surgery 16 and 17 years after the presentation of tumor, which was successfully removed (22).
In the Cohen’s report, a patient showed metastasis in the occipital lobe and cerebellum. Metastasis appeared seven months after the primary tumor treatment (23). Few patients (only four cases) had died at the time of report. Therefore, the ultimate outcome was difficult to conclude. The median DFS was 24 months. Also, the five- and ten - year DFS rates were 50.1% and 25.0%, respectively. The median overall survival was 24 months, and the five-year and ten - year overall survival was 63.8%.
4.1. Conclusion
PTPR was found in all age groups, with a generally indolent course. It seems that local recurrence is the main cause of treatment failure in PTPR. After recurrence, various clinical courses were found, which warrant further investigation. Overall, a combination of treatments may be more beneficial for patients. It seems that adjuvant RT and chemotherapy are more important than a comprehensive surgery.
Acknowledgements
References
-
1.
Li J, Recinos PF, Orr BA, Burger PC, Jallo GI, Recinos VR. Papillary tumor of the pineal region in a 15-month-old boy. J Neurosurg Pediatr. 2011;7(5):534-8. [PubMed ID: 21529195]. [PubMed Central ID: PMC4612618]. https://doi.org/10.3171/2011.2.PEDS10434.
-
2.
Jouvet A, Fauchon F, Liberski P, Saint-Pierre G, Didier-Bazes M, Heitzmann A, et al. Papillary tumor of the pineal region. Am J Surg Pathol. 2003;27(4):505-12. [PubMed ID: 12657936].
-
3.
Shibahara J, Todo T, Morita A, Mori H, Aoki S, Fukayama M. Papillary neuroepithelial tumor of the pineal region. A case report. Acta Neuropathol. 2004;108(4):337-40. [PubMed ID: 15221340]. https://doi.org/10.1007/s00401-004-0898-z.
-
4.
Castaneyra-Perdomo A, Meyer G, Ferres-Torres R. The early development of the human subcommissural organ. J Anat. 1985;143:195-200. [PubMed ID: 3870727]. [PubMed Central ID: PMC1166438].
-
5.
Rodriguez EM, Rodriguez S, Hein S. The subcommissural organ. Microsc Res Tech. 1998;41(2):98-123. https://doi.org/10.1002/(sici)1097-0029(19980415)41:2<98::aid-jemt2>3.0.co;2-m.
-
6.
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97-109. [PubMed ID: 17618441]. [PubMed Central ID: PMC1929165]. https://doi.org/10.1007/s00401-007-0243-4.
-
7.
Fevre Montange M, Vasiljevic A, Champier J, Jouvet A. Papillary tumor of the pineal region: Histopathological characterization and review of the literature. Neurochirurgie. 2015;61(2-3):138-42. [PubMed ID: 24556386]. https://doi.org/10.1016/j.neuchi.2013.04.011.
-
8.
Cykowski MD, Wartchow EP, Mierau GW, Stolzenberg ED, Gumerlock MK, Fung KM. Papillary tumor of the pineal region: ultrastructural study of a case. Ultrastruct Pathol. 2012;36(1):68-77. [PubMed ID: 22292738]. https://doi.org/10.3109/01913123.2011.620222.
-
9.
Riis P, van Eck AT, Dunker H, Bergmann M, Borm W. Stereotactic radiosurgery of a papillary tumor of the pineal region: case report and review of the literature. Stereotact Funct Neurosurg. 2013;91(3):186-9. [PubMed ID: 23446182]. https://doi.org/10.1159/000344023.
-
10.
Cerase A, Vallone IM, Di Pietro G, Oliveri G, Miracco C, Venturi C. Neuroradiological follow-up of the growth of papillary tumor of the pineal region: a case report. J Neurooncol. 2009;95(3):433-5. [PubMed ID: 19517065]. https://doi.org/10.1007/s11060-009-9931-3.
-
11.
Santarius T, Joseph JA, Tsang KT, O'Donovan DG, Kirollos RW. Papillary tumour of the pineal region. Br J Neurosurg. 2008;22(1):116-20. [PubMed ID: 17891572]. https://doi.org/10.1080/02688690701583794.
-
12.
Santoro A, D'Elia A, Fazzolari B, Santoro F, Antonelli M, Giangaspero F, et al. Four-year clinical and neuroradiological follow-up of a papillary tumor of the pineal region. Neurol Sci. 2012;33(4):931-5. [PubMed ID: 22124853]. https://doi.org/10.1007/s10072-011-0860-5.
-
13.
Cohan JN, Moliterno JA, Mok CL, Lavi E, Boockvar JA. Pineal parenchymal tumor of intermediate differentiation with papillary features: a continuum of primary pineal tumors? J Neurooncol. 2011;101(2):301-6. [PubMed ID: 20521161]. https://doi.org/10.1007/s11060-010-0242-5.
-
14.
Rickard KA, Parker JR, Vitaz TW, Plaga AR, Wagner S, Parker JJ. Papillary tumor of the pineal region: two case studies and a review of the literature. Ann Clin Lab Sci. 2011;41(2):174-81. [PubMed ID: 21844577].
-
15.
Sato TS, Kirby PA, Buatti JM, Moritani T. Papillary tumor of the pineal region: report of a rapidly progressive tumor with possible multicentric origin. Pediatr Radiol. 2009;39(2):188-90. [PubMed ID: 19037636]. https://doi.org/10.1007/s00247-008-1060-1.
-
16.
Epari S, Bashyal R, Malick S, Gupta T, Moyadi A, Kane SV, et al. Papillary tumor of pineal region: report of three cases and review of literature. Neurol India. 2011;59(3):455-60. [PubMed ID: 21743183]. https://doi.org/10.4103/0028-3886.82773.
-
17.
Hua X, Yang P, Zhang M, Zhao Y, Wang B. Papillary tumor of the pineal region: A case report and review of the literature. Exp Ther Med. 2015;10(4):1375-9. [PubMed ID: 26622493]. [PubMed Central ID: PMC4578110]. https://doi.org/10.3892/etm.2015.2696.
-
18.
Kawahara I, Tokunaga Y, Yagi N, Iseki M, Abe K, Hayashi T. Papillary Tumor of the Pineal Region. Neurologia medico-chirurgica. 2007;47(12):568-71. https://doi.org/10.2176/nmc.47.568.
-
19.
Sun IS, Kirollos R, Santarius T. No 5-ALA fluorescence seen in a recurrent papillary tumour of the pineal region (PTPR). Acta Neurochir (Wien). 2015;157(2):215-6. [PubMed ID: 25520052]. https://doi.org/10.1007/s00701-014-2308-1.
-
20.
Gutenberg A, Brandis A, Hong B, Gunawan B, Enders C, Schaefer IM, et al. Common molecular cytogenetic pathway in papillary tumors of the pineal region (PTPR). Brain Pathol. 2011;21(6):672-7. [PubMed ID: 21470326]. https://doi.org/10.1111/j.1750-3639.2011.00493.x.
-
21.
Yano H, Ohe N, Nakayama N, Shinoda J, Iwama T. Clinicopathological features from long-term observation of a papillary tumor of the pineal region (PTPR): a case report. Brain Tumor Pathol. 2009;26(2):83-8. [PubMed ID: 19856220]. https://doi.org/10.1007/s10014-009-0246-z.
-
22.
Lechapt-Zalcman E, Chapon F, Guillamo JS, Khouri S, Menegalli-Boggelli D, Loussouarn D, et al. Long-term clinicopathological observations on a papillary tumour of the pineal region. Neuropathol Appl Neurobiol. 2011;37(4):431-5. [PubMed ID: 20942871]. https://doi.org/10.1111/j.1365-2990.2010.01133.x.
-
23.
Cohen AL, Salzman K, Palmer C, Jensen R, Colman H. Bevacizumab is Effective for Recurrent Papillary Tumor of the Pineal Region: First Report. Case Rep Oncol. 2013;6(2):434-40. [PubMed ID: 24019784]. [PubMed Central ID: PMC3764969]. https://doi.org/10.1159/000354753.
-
24.
Chatterjee D, Gupta K, Kumar N, Chhabra R, Radotra BD. Papillary tumor of the pineal region-report of three cases with literature review. Neurol India. 2015;63(4):567-70. [PubMed ID: 26238893]. https://doi.org/10.4103/0028-3886.162055.
-
25.
Shakir HJ, Qiu J, Prasad D, Mechtler LL, Fenstermaker RA. Papillary tumor of the pineal region with extended clinical and radiologic follow-up. Surg Neurol Int. 2015;6(Suppl 18):S451-4. [PubMed ID: 26539320]. [PubMed Central ID: PMC4604644]. https://doi.org/10.4103/2152-7806.166782.
-
26.
Patel SK, Tomei KL, Christiano LD, Baisre A, Liu JK. Complete regression of papillary tumor of the pineal region after radiation therapy: case report and review of the literature. J Neurooncol. 2012;107(2):427-34. [PubMed ID: 22086239]. https://doi.org/10.1007/s11060-011-0764-5.
-
27.
Nakamura H, Makino K, Kochi M, Nakazato Y, Kuratsu J. Successful treatment of neoadjuvant therapy for papillary tumor of the pineal region. Brain Tumor Pathol. 2009;26(2):73-7. [PubMed ID: 19856218]. https://doi.org/10.1007/s10014-009-0250-3.
-
28.
Dagnew E, Langford LA, Lang FF, DeMonte F. Papillary tumors of the pineal region: case report. Neurosurgery. 2007;60(5):E953-5. discussion E953-5. [PubMed ID: 17460510]. https://doi.org/10.1227/01.NEU.0000255443.44365.77.
-
29.
Inoue T, Kumabe T, Kanamori M, Sonoda Y, Watanabe M, Tominaga T. Papillary tumor of the pineal region: a case report. Brain Tumor Pathol. 2008;25(2):85-90. [PubMed ID: 18987834]. https://doi.org/10.1007/s10014-008-0231-y.
-
30.
Kim YH, Kim JW, Park CK, Kim DG, Sohn CH, Chang KH, et al. Papillary tumor of pineal region presenting with leptomeningeal seeding. Neuropathology. 2010;30(6):654-60. [PubMed ID: 20374498]. https://doi.org/10.1111/j.1440-1789.2010.01108.x.
-
31.
Ishida A, Shibuya M, Komori T, Nobusawa S, Niimura K, Matsuo S, et al. Papillary tumor of the pineal region: a case involving isocitrate dehydrogenase (IDH) genotyping. Brain Tumor Pathol. 2013;30(1):45-9. [PubMed ID: 22466620]. https://doi.org/10.1007/s10014-012-0098-9.
-
32.
Hong B, Nakamura M, Brandis A, Becker H, Krauss JK. Spinal metastasis of papillary tumor of the pineal region. Clin Neurol Neurosurg. 2011;113(3):235-8. [PubMed ID: 21109346]. https://doi.org/10.1016/j.clineuro.2010.10.010.
-
33.
Cardenas R, Javalkar V, Haydel J, Wadhwa R, Fowler M, Scheithauer B, et al. Papillary tumor of pineal region: prolonged control rate after gamma knife radiosurgery - a case report and review of literature. Neurol India. 2010;58(3):471-6. [PubMed ID: 20644284]. https://doi.org/10.4103/0028-3886.66051.
-
34.
El Majdoub F, Blau T, Hoevels M, Buhrle C, Deckert M, Treuer H, et al. Papillary tumors of the pineal region: a novel therapeutic option-stereotactic 125iodine brachytherapy. J Neurooncol. 2012;109(1):99-104. [PubMed ID: 22528796]. https://doi.org/10.1007/s11060-012-0870-z.
-
35.
Lorenzetti M, Motta F, Campanella R, Bauer D, Assi A, Arienta C, et al. Adjuvant temozolomide chemotherapy for treatment of papillary tumor of the pineal region. World Neurosurg. 2011;76(1-2):160-3. [PubMed ID: 21839968]. https://doi.org/10.1016/j.wneu.2010.10.039.
-
36.
Nowicka E, Bobek-Billewicz B, Szymas J, Tarnawski R. Late dissemination via cerebrospinal fluid of papillary tumor of the pineal region: a case report and literature review. Folia Neuropathol. 2016;54(1):72-9. [PubMed ID: 27179224].
-
37.
Koziarski A, Grala B, Skrobowska E. Papillary tumor of the pineal region. Report of two cases and literature review. Neurol Neurochir Pol. 2014;48(5):356-62. [PubMed ID: 25440015]. https://doi.org/10.1016/j.pjnns.2014.09.005.