Abstract
Background:
Accelerated repopulation in HNSCC (head and neck squamous cell cancer) is responsible for poor outcomes; which can be tackled either by hyperfractionation or hypofractionation. Multiple daily fractionations in a setting with more patient load is cumbersome; hence we have tried to escalate the dose to the tumor with SIB by increasing the dose per fraction in the last two weeks of treatment to the tumor; thus, trying to achieve better locoregional control within a shorter period of treatment time.Methods:
A total of 20 histologically proven LAHNSCC patients, enrolled alternatively into the control arm (GTV 66Gy, CTVhigh risk 60Gy, and CTVlow risk 54Gy) and the study arm (32Gy to the GTV, CTVhigh risk, CTVlow risk in 16 fractions followed by 30Gy to the GTV, 25Gy to the CTVhigh risk and 20 Gy to CTVlow risk in 10 fractions,), in both arm patients received one fraction per day, five days per week. The radiological response assessment was done using a CT scan at the end of one month after treatment. Toxicity assessment was done weekly during treatment, at the end of treatment, in the first month, and at the end of two months.Results:
In this study the patients completed treatment with 26 fractions in about five to six weeks with Grade 3 mucositis in 90% and grade 3 dysphagia in 40% of the patients, which necessitated Ryle’s tube feeding in 30% of the with complete resolution of the mucositis and to grade 1 dysphagia by the end of three months. Grade 1 xerostomia was noted in all the patients with gradual resolution of symptoms. The overall complete response (CR) was achieved in 50% of the patients and the CR with reference to the tumor was seen in 80% and with reference to the node was seen in 50% of the patients, respectively.Conclusions:
IMRT with Hypofractionated concomitant simultaneous integrated boost (SIB) was better than standard IMRT with SIB in LAHNSCC, with respect to radiological response, however, at the cost of higher toxicity.Keywords
Simultaneous Integrated Boost IMRT Hypofractionation Head and Neck Cancers
1. Background
Head and neck cancer accounts for about one-third of all cancers in India. According to the Indian Council of Medical Research, approximately 0.2 to 0.25 million new head and neck cancer patients are diagnosed each year (1).
The global number of new cancers of the oral cavity, nasopharynx, and other pharyngeal sites have been estimated to be 455000. Annually, these tumors are responsible for over 300000 cancer deaths (2).
Overall 57.5% of global head and neck cancers occur in Asia, especially in India. In India 60% to 80% of patients are present with the advanced disease, in comparison with 40% in developed countries (3).
The treatment modalities used in the treatment of head and neck cancer are surgery, radiotherapy, and chemotherapy. Organ-preservation protocols using combined chemoradiation has become the standard of care for locally advanced oropharyngeal, hypopharyngeal, and laryngeal carcinomas. The very advanced hypopharyngeal and laryngeal carcinomas are treated by surgery, followed by postoperative chemoradiation (4).
Radiation therapy has been the standard of care for unresectable LAHNSCC patients, although the overall survival after radiation has, in general, been less than 25%. A number of efforts have been made to improve these disappointing results, including altered radiation therapy fractionation schedules and the use of systemic chemotherapy in conjunction with radiation (5). The survival rates for patients with advanced disease (stage III - IV) is in the 30% - 40% range, and the majority of these patients will eventually die of cancer (6). Due to locoregional recurrence of cancer, it is of utmost importance to develop treatment protocols that are able to produce maximal local control, which can be achieved with different fractionation schedules like hyperfractionated and accelerated radiotherapy (7).
More intense combination therapy leads to intensification of acute radiation- and chemotherapy related adverse events in normal tissues like acute radiation-related mucosal reactions, which make patients more vulnerable to infections, malnutrition, and disruptions in the course of radiotherapy, which can lead to inferior local control (8, 9). The delayed effects of radiotherapy, such as radiation induced xerostomia, can be distressing for patients. This can be reduced with current conformal radiotherapeutic techniques like intensity-modulated radiotherapy (IMRT), which enable escalating the radiotherapy doses given to advanced tumors and simultaneously reducing the doses to healthy normal tissues, thus, significantly improving the therapeutic ratio of radiotherapy (10). Currently the standard is IMRT with chemotherapy. SIB/SMART (simultaneous modulated accelerated radiation therapy) is an accepted mode of delivery of IMRT.
The issue of accelerated repopulation in squamous cell Head and neck carcinoma can be addressed by accelerating the RT, which can be done by hyperfractionation or hypofractionation. The former has been demonstrated by MD Anderson hyperfractionated RT with concomitant boost. The latter is being evaluated by us in a case control study by comparing SIB standard with hypofractionated accelerated radiotherapy with SIB, the BED being comparable in both the arms with GTV, CTVhigh risk, CTVlow risk having a BED of 74.5 Gy, 66 Gy, 57.7 Gy in the standard SIB arm, and 72.4 Gy, 64.7 Gy, 57.4 Gy in the study arm, respectively.
As multiple daily fractionations are not feasible for many institutions: hypofractionated accelerated RT is less labor intensive due to the fact that instead of accelerating by increasing fractionation we increase the dose per fraction. High toxicity of the surrounding normal tissue is counteracted by IMRT. Neck nodes can be targeted simultaneously and dose delivered to the nodes can be increased.
2. Methods
2.1. Eligibility Criteria
The study protocol and consent procedure were approved by the Institutional Ethical Committee situated at Kidwai Memorial Institute of Oncology, M. H. Marigowda Road, Bangalore, 560029 Karnataka, India. Patients aged between 18 - 75 years with KPS > 70, with a histological diagnosis of squamous cell carcinoma of head neck stage III - IV A, with no history of surgery of primary tumor, prior radiation therapy were eligible. Patient characteristics are depicted in Table 1.
Patient Characteristics
| Hypo Fractionated SIB Arm, No. (%) | SIB Arm, No. (%) | P Value | |
|---|---|---|---|
| Total | 10 (100) | 10 (100) | |
| Age | 0.038 | ||
| 40 - 50 | 5 (50) | 0 (0) | |
| 51 - 60 | 3 (30) | 8 (80) | |
| 61 - 70 | 2 (20) | 1 (10) | |
| > 70 | 0 (0) | 1 (10) | |
| Gender | 1 | ||
| Male | 9 (90) | 10 (100) | |
| Female | 1 (10) | 0 (0) | |
| KPS (Karnofsky performance status) | 1 | ||
| 80 | 4 (40) | 3 (30) | |
| 90 | 6 (60) | 7 (70) | |
| Site of primary | 0.246 | ||
| Base of tongue | 3 (30) | 2 (20) | |
| Supraglottis | 0 (0) | 3 (30) | |
| Tonsil | 5 (50) | 2 (20) | |
| Vallecula | 2 (20) | 3 (30) | |
| Tumor stage | |||
| T1 | 0 (0) | 0 (0) | |
| T2 | 0 (0) | 1 (10) | |
| T3 | 9 (90) | 7 (70) | |
| T4a | 1 (10) | 2 (20) | |
| Nodal stage | 0.735 | ||
| N0 | 4 (40) | 2 (20) | |
| N1 | 3 (30) | 4 (40) | |
| N2 | 3 (30) | 4 (40) | |
| Stage | 1 | ||
| I | 0 (0) | 0 (0) | |
| II | 0 (0) | 0 (0) | |
| III | 7 (70) | 6 (60) | |
| IV A | 3 (30) | 4 (40) | |
| Overall treatment time (days) | < 0.001 | ||
| 40 - 45 | 9 (90) | 0 (0) | |
| 46 - 50 | 1 (10) | 7 (70) | |
| 51 - 55 | 0 (0) | 3 (30) |
After obtaining informed consent, patients were alternatively enrolled into control arm and study arm.
2.2. Radiation Treatment
Patients were treated with 6 MV X-rays on IMRT using SIB technique to give a total EQD2 of 70 Gy to GTV, 60 Gy to CTVhigh risk, 50 Gy to CTVlow risk in both arms.
2.2.1. Patients in the Control Arm
The gross tumor and lymph node metastasis including non-palpable lymph nodes suspicious for metastasis, according to radiologic criteria (primary PTVs), received 30 fractions of 2.2 Gy/fraction to a total dose of 66 Gy. Subclinical PTV60, at high risk (first echelon nodes and the high-risk area), received 30 fractions of 2.0 Gy/fraction to a total dose of 60 Gy. Subclinical disease (PTV54) received 30 fractions of 1.8 Gy/fraction to a dose of 54 Gy.
Treatment was delivered once daily, five fractions per week, over 6 weeks.
2.2.2. Patients in the Study Arm
32 Gy to the PTV corresponding to GTV, CTVhigh risk, CTVlow risk in 16 fractions,1 fraction per day, five days a week followed by 30 Gy to the PTV to GTV, 25 Gy to the PTV to CTVhigh risk and 20 Gy to the PTV to CTVlow risk in 10 fractions, 1 fraction per day with 5 fractions/week.
A quality assurance program used a dosimetric check of all IMRT fields prior to treatment using point dose/IMATRIX. Treatment execution was done by electronic portal imaging devices (EPID).
2.3. Radiological Response Assessment
All patients underwent a CT scan at the end of one month after treatment and response assessment was done as per the WHO Tumor Response Criteria and the results are depicted in Tables 2 - 4.
2.4. Toxicity
Toxicity assessment was done weekly during treatment, at the end of treatment, in the first month, and at the end of two months by RTOG toxicity profile; the results are depicted in Tables 5 - 8.
3. Results
Our study is a comparative two groups parallel design:
| Response at the End of Treatment | Hypo Fractionated SIB Arm (N = 10) | SIB Arm (N = 10) | Total (N = 20) | P Value |
|---|---|---|---|---|
| T | 0.650 | |||
| CR (complete response) | 7 (70) | 5 (50) | 12 (60) | |
| PR (partial response) | 3 (30) | 5 (50) | 8 (40) | |
| N | 0.289 | |||
| CR | 4 (40) | 4 (40.0) | 8 (40.0) | |
| N/A | 2 (20) | 5 (50.0) | 7 (35.0) | |
| PR | 4 (40) | 1 (10) | 5 (25.0) |
| Response at the End of 1 Month | Hypo Fractionated SIB Arm (N = 10) | SIB Arm (N = 10) | Total (N = 20) | P Value |
|---|---|---|---|---|
| T | 0.628 | |||
| CR | 8 (80) | 6 (60) | 14 (70) | |
| PR | 2 (20) | 4 (40) | 6 (30) | |
| N | 0.714 | |||
| CR | 5 (50) | 4 (40) | 9 (45) | |
| N/A | 2 (20) | 1 (10) | 3 (15) | |
| PR | 3 (30) | 5 (50) | 8 (40) |
Overall Response
| Overall Response | Hypo Fractionated SIB Arm | SIB Arm | Total | P Value |
|---|---|---|---|---|
| CR | 5 (50) | 4 (40) | 9 (45) | 1.000 |
| PR | 5 (50) | 6 (60) | 11 (55) | |
| Total | 10 (100) | 10 (100) | 20 (100) |
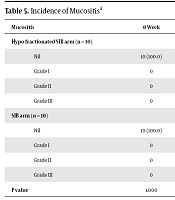
| Mucositis | 0 Week | 1 Week | 2 Weeks | 3 Weeks | 4 Weeks | 5 Weeks | 6 Weeks | 7 Weeks | 1 Month | 3 Months |
|---|---|---|---|---|---|---|---|---|---|---|
| Hypo fractionated SIB arm (n = 10) | ||||||||||
| Nil | 10 (100.0) | 10 (100.0) | 8(80.0) | 0 | 0 | 0 | 0 | 0 | 4 (40.0) | 10 (100.0) |
| Grade I | 0 | 0 | 1 (10.0) | 1 (10.0) | 0 | 0 | 0 | 0 | 0 | 0 |
| Grade II | 0 | 0 | 1 (10.0) | 9 (90.0) | 9 (90.0) | 5 (50.0) | 1 (10.0) | 1 (10.0) | 6 (60.0) | 0 |
| Grade III | 0 | 0 | 0 | 0 | 1 (10.0) | 5 (50.0) | 9 (90.0) | 9 (90.0) | 0 | 0 |
| SIB arm (n = 10) | ||||||||||
| Nil | 10 (100.0) | 10 (100.0) | 7 (70.0) | 4 (40.0) | 0 | 0 | 0 | 0 | 5 (50.0) | 10 (100.0) |
| Grade I | 0 | 0 | 0 | 3 (30.0) | 3 (30.0) | 1 (10.0) | 0 | 0 | 4 (40.0) | 0 |
| Grade II | 0 | 0 | 3 (30.0) | 3 (30.0) | 6 (60.0) | 8 (80.0) | 7 (70.0) | 7 (70.0) | 1 (10.0) | 0 |
| Grade III | 0 | 0 | 0 | 0 | 1 (10.0) | 1 (10.0) | 3 (30.0) | 3 (30.0) | 0 | 0 |
| P value | 1.000 | 1.000 | 0.582 | 0.020* | 0.020* | 0.141 | 0.020* | 0.020* | 0.017* | 1.000 |
| Dysphagia | 0 Week | 1 Week | 2 Weeks | 3 Weeks | 4 Weeks | 5 Weeks | 6 Weeks | 7 Weeks | 1 Month | 3 Months |
|---|---|---|---|---|---|---|---|---|---|---|
| Hypo fractionated SIB arm (n = 10) | ||||||||||
| Nil | 10 (100.0) | 10 (100.0) | 8 (80.0) | 4 (40.0) | 2 (20.0) | 0 | 0 | 0 | 3 (30.0) | 6 (60.0) |
| Grade I | 0 | 0 | 2 (20.0) | 6 (60.0) | 4 (40.0) | 2 (20.0) | 0 | 0 | 3 (30.0) | 4 (40.0) |
| Grade II | 0 | 0 | 0 | 0 | 4 (40.0) | 5 (50.0) | 6 (60.0) | 5 (50.0) | 4 (40.0) | 0 |
| Grade III | 0 | 0 | 0 | 0 | 0 | 3 (30.0) | 4 (40.0) | 5 (50.0) | 0 | 0 |
| SIB Arm (n = 10) | ||||||||||
| Nil | 10 (100.0) | 10 (100.0) | 5 (50.0) | 3 (30.0) | 0 | 0 | 0 | 0 | 2 (20.0) | 9 (90.0) |
| Grade I | 0 | 0 | 5 (50.0) | 6 (60.0) | 4 (40.0) | 3 (30.0) | 0 | 0 | 8 (80.0) | 1 (10.0) |
| Grade II | 0 | 0 | 0 | 1 (10.0) | 6 (60.0) | 7 (70.0) | 7 (70.0) | 6 (60.0) | 0 | 0 |
| Grade III | 0 | 0 | 0 | 0 | 0 | 0 | 3 (30.0) | 4 (40.0) | 0 | 0 |
| P value | 1.000 | 1.000 | 0.350 | 1.000 | 0.554 | 0.314 | 1.000 | 1.000 | 0.053+ | 0.303 |
| Skin Toxicity | 0 Week | 1 Week | 2 Weeks | 3 Weeks | 4 Weeks | 5 Weeks | 6 Weeks | 7 Weeks | 1 Month | 3 Months |
|---|---|---|---|---|---|---|---|---|---|---|
| Hypo fractionated SIB arm (n = 10) | ||||||||||
| Nil | 10 (100) | 10 (100.0) | 10 (100.0) | 9 (90.0) | 7 (70.0) | 7 (70.0) | 5 (50.0) | 7 (70.0) | 10 (100.0) | 10 (100.0) |
| Grade I | 0 | 0 | 0 | 1 (10.0) | 3 (30.0) | 3 (30.0) | 5 (50.0) | 2 (30.0) | 0 | 0 |
| Grade II | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (10.0) | 0 | 0 |
| Grade III | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| SIB Arm (n = 10) | ||||||||||
| Nil | 10 (100.0) | 10 (100.0) | 10 (100.0) | 10 (100.0) | 9 (90.0) | 7 (70.0) | 4 (40.0) | 5 (50.0) | 10 (100.0) | 10 (100.0) |
| Grade I | 0 | 0 | 0 | 0 | 1 (10.0) | 3 (30.0) | 6 (60.0) | 5 (50.0) | 0 | 0 |
| Grade II | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Grade III | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| P value | 1.000 | 1.000 | 1.000 | 1.000 | 0.582 | 1.000 | 1.000 | 0.650 | 1.000 | 1.000 |
| Xerostomia Grade | Hypo Fractionated SIB Arm (N = 10) | SIB Arm (N = 10) | Total (N = 20) | P Value |
|---|---|---|---|---|
| 1 month | 1.000 | |||
| Nil | 0 (0) | 0 (0) | 0 (0) | |
| Grade I | 10 (100) | 10 (100) | 20 (100) | |
| Grade II | 0 (0) | 0 (0) | 0 (0) | |
| 3 months | 0.474 | |||
| Nil | 0 (0) | 2 (20) | 2 (10) | |
| Grade I | 10 (100) | 8 (80) | 18 (90) | |
| Grade II | 0 (0) | 0 (0) | 0 (0) |
4. Discussion
The present study has been taken up to tackle the issue of accelerated repopulation in LAHNSCC, which can be done either by hyperfractionation as shown by the MD Anderson hyperfractionated RT with concomitant boost or with hypofractionation.
Gwozdz et al., in the MD Anderson concomitant boost radiotherapy for squamous cell carcinoma of the tonsillar fossa showed high rates of local and regional disease control with the concomitant boost fractionation schedule, with few cases of severe late morbidity. Of the 83 patients, about five patients had Gr 4 confluent mucositis. The acute mucositis was resolved in all cases. The five year actuarial local control rate of patients who received the boost during the final treatment phase was 87%. The five year actuarial loco regional control rate overall was 77%. The loco regional control rates for patients with AJCC stages II, III, and IV disease were 76, 65, and 85%, respectively (11).
Multiple daily fractionation in a setting with more patient load is cumbersome and not feasible. Thus, the present study was intended to escalate the dose to the tumor with SIB by increasing the dose per fraction in the last two weeks of treatment to the tumor; thus, trying to achieve better locoregional control within a shorter period of time and also to prevent treatment interruptions as the toxicity of normal tissues like mucositis is likely to peak toward the end or after the treatment, thereby ensuring better compliance.
In the present study, with respect to toxicity, there was grade 3 mucositis in 90% and grade 3 dysphagia in 40% of the patients in the study arm, which necessitated ryles tube feeding in 30% of the patients, but with complete resolution of the mucositis and to grade 1 dysphagia by the end of 3 months.
With the mean dose to the parotids being in the range of 28 to 31 Gy the xerostomia noted was also grade 1 in all the patients with gradual resolution of symptoms.
The overall complete response was achieved in 50% of the patients of the study arm, 40% of the patients in control arm; the complete response with reference to the tumor was seen in 80% of the patients of the study arm, 60% of the patients in control arm, and with reference to the node, complete response was seen in 50% of the patients of the study arm and 40% of the patients in control arm. However, these differences were not statistically significant.
The present study showed that dose escalation in the last two weeks of treatment is tolerated by the patients with acute normal tissue complications peaking close to the end of treatment with resolution of the toxicities by the end of 3 months’ post treatment. In addition, with evolution of functional imaging, it will be possible to identify the areas of interest where dose escalation is needed. One of the short-comings of our study is small number of patients. Hence this needs to be confirmed in a study with a larger population.
4.1. Conclusions
IMRT with Hypofractionated concomitant simultaneous integrated boost (SIB) was better than Standard IMRT with SIB in LAHNSCC, with respect to radiological response, however, at the cost of higher toxicity.
References
-
1.
Trivedi NP, Kekatpure VD, Trivedi NN, Kuriakose MA. Head and neck cancer in India: Need to formulate uniform national treatment guideline? Indian J Cancer. 2012;49(1):6-10. [PubMed ID: 22842161]. https://doi.org/10.4103/0019-509X.98907.
-
2.
Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2(9):533-43. [PubMed ID: 11905707]. https://doi.org/10.1016/S1470-2045(01)00486-7.
-
3.
Kulkarni MR. Head and neck cancer burden in India. Int J Head Neck Surg. 2013;4(1):29-35. https://doi.org/10.5005/jp-journals-10001-1132.
-
4.
Braakhuis BJ, Brakenhoff RH, Leemans CR. Treatment choice for locally advanced head and neck cancers on the basis of risk factors: Biological risk factors. Ann Oncol. 2012;23 Suppl 10:x173-7. [PubMed ID: 22987957]. https://doi.org/10.1093/annonc/mds299.
-
5.
Adelstein DJ, Li Y, Adams GL, Wagner H Jr, Kish JA, Ensley JF, et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol. 2003;21(1):92-8. [PubMed ID: 12506176]. https://doi.org/10.1200/JCO.2003.01.008.
-
6.
Vokes EE, Weichselbaum RR, Lippman SM, Hong WK. Head and neck cancer. N Engl J Med. 1993;328(3):184-94. [PubMed ID: 8417385]. https://doi.org/10.1056/NEJM199301213280306.
-
7.
Fu KK, Pajak TF, Trotti A, Jones CU, Spencer SA, Phillips TL, et al. A radiation therapy oncology group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: First report of RTOG 9003. International Journal of Radiation Oncology*Biology*Physics. 2000;48(1):7-16. https://doi.org/10.1016/s0360-3016(00)00663-5.
-
8.
Maciejewski B, Withers HR, Taylor JM, Hliniak A. Dose fractionation and regeneration in radiotherapy for cancer of the oral cavity and oropharynx: Tumor dose-response and repopulation. Int J Radiat Oncol Biol Phys. 1989;16(3):831-43. [PubMed ID: 2921175]. https://doi.org/10.1016/0360-3016(89)90503-8.
-
9.
Fowler JF, Lindstrom MJ. Loss of local control with prolongation in radiotherapy. Int J Radiat Oncol Biol Phys. 1992;23(2):457-67. [PubMed ID: 1534082]. https://doi.org/10.1016/0360-3016(92)90768-d.
-
10.
Intensity Modulated Radiation Therapy Collaborative Working Group. Intensity-modulated radiotherapy: Current status and issues of interest. Int J Radiat Oncol Biol Phys. 2001;51(4):880-914. [PubMed ID: 11704310]. https://doi.org/10.1016/s0360-3016(01)01749-7.
-
11.
Gwozdz JT, Morrison WH, Garden AS, Weber RS, Peters LJ, Ang KK. Concomitant boost radiotherapy for squamous carcinoma of the tonsillar fossa. Int J Radiat Oncol Biol Phys. 1997;39(1):127-35. https://doi.org/10.1016/s0360-3016(97)00291-5.