Abstract
Introduction:
In this paper, we present the first experience of linac-based stereotactic body radiation therapy (SBRT) in Iran to treat a single liver metastasis.Case Presentation:
A 23-year-old girl with a history of visceral melanoma of the uterine was treated for liver metastasis. Stereotactic body radiotherapy was selected as the treatment of choice after tumor recurrence following surgical removal and radiofrequency ablation. The treatment was delivered in five fractions to a total dose of 50 Gy. The patient tolerated the treatment without any considerable side effect and the lesion remained progression-free twelve months after treatment.Conclusions:
It can be presumed that SBRT can be safely applied using IGRT-equipped conventional linear accelerators with a few but no adverse events if it is delivered with due consideration.Keywords
1. Introduction
Modern technologic advancements in medicine have led to the opening of new paradigms in cancer treatment. Stereotactic body radiotherapy (SBRT) (1) is one of the promising treatment techniques made possible by the introduction of new technologies in the era of radiation therapy. By definition, this technique involves highly accurate delivery of a high radiation dose using external beam radiotherapy (EBRT) in a few treatment sessions. Stereotactic body radiotherapy has been used to treat primary or oligometastatic lesions in various parts of the body, including the lung, liver, bone, etc. (1, 2). Stereotactic body radiotherapy of limited metastatic lesions has piloted a concept of cure in these patients (3). The delivery of moderate to extreme radiation doses can be done with SBRT dedicated machines such as CyberKnife or C-arm linacs used for conventional treatments. Commissioning and safe implementation of C-arm linac-based SBRT are addressed in accredited guidelines (4). Organ motion related to the breathing cycle needs to be taken into account for tumors located in thoracic or abdominal regions. Technical strategies to counteract organ motion during breathing cycles essentially include the application of gating systems. However, in some circumstances, treatment in the free-breathing phase with consideration of the maximal movement of pertaining organs is not only more tolerable but also reasonably acceptable (5). Tumor characteristics such as size and location are major factors to select motion management strategy and dose fractionation regimen. Selecting eligible patients to treat during free-breathing cycles is crucial so that the maximum breathing movement of target organs falls below certain limits (6)
In the meantime, image guidance with either portal images in the presence of tumor localizing fiducial markers or cone-beam CT scans (CBCT) obtained in the treatment room is obligatory to localize targets and surrounding organs at risk and to minimize random errors due to positioning and daily setup variations.
The liver is one of the most common sites for metastases from melanoma as one of the most aggressive forms of skin and mucosal cancer. In spite of recent advances in the systemic treatment of metastatic malignant melanoma after the introduction of immunotherapy drugs, surgical resection of single metastatic lesions has remained the most effective method to achieve durable control. Liver capacity for regeneration is high enough to compensate for damages after surgery or other ablative treatments such as radiofrequency or SBRT. The melanoma tumor cell proliferation rate is low and its alpha-beta ratio is relatively low consequently (7). Melanoma is a good candidate for SBRT with a high dose per fraction delivery. Therefore, SBRT can be selected based on the discretion of physicians and in the case of previously failed Radiofrequency Ablation (RFA) treatment and the patient’s unwillingness for further surgical interventions.
In this case study, we present the first experience of linac-based SBRT in free-breathing for liver melanoma metastasis, which could be used as a reference for future applications. We adopted a five-fraction schedule according to AAPM Task Group 1013 and the SPARC protocol (8).
2. Case Presentation
The patient was a 23-year-old athletic female who first presented in July 2013 after being diagnosed with melanoma aroused in the lower segment of her uterus. The patient underwent trans-abdominal hysterectomy and bilateral salpingo-oophorectomy. Pathologic stage III was diagnosed due to the extension of the tumor to regional lymph nodes. Adjuvant whole pelvis external beam radiotherapy was prescribed in 25 fractions to a total dose of 50 Gy using the 3-D conformal box technique. We also prescribed PEG-interferon for the patient but it discontinued just after the first administration due to the patient’s intolerance.
In February 2015, follow-up imaging revealed two metastatic lesions in segment IV and VII of the liver confirmed by a biopsy. However, FDG-PET/CT scan revealed no other metastasis. She was treated with radiofrequency ablation that resulted in the complete resolution of the lesions after five months. In June 2017, the disease recurred adjacent to the previous location in segment V of the liver and and was surgically removed with free margins. In August 2018, a 40-mm lesion in the right liver lobe adjacent to the previous surgery site was noted in CT scans, indicative of recurrent disease. Regarding the past surgical history and ablation treatment and concerning the patient’s refusal for further surgical resection, she opted for SBRT of the liver metastasis. The patient was not a good candidate for targeted therapy and immunotherapy due to the lack of tumor V600E driving mutations and low expression of PDL1. We presented our ability and the protocol for this treatment in detail. Verbal consent was obtained after discussing the benefits and disadvantages of SBRT with the patient and her parents.
2.1. Simulation
We used two series of three markers (left, right, and midline) on the patient’s skin in the treatment position (supine position with arms over the head) to check setup accuracy during treatment sessions. The first series of markers was put at the xiphoid level and the second series was put 10 cm above the xiphoid using the same setup laser beams. All markers were tattooed on the patient’s skin. A contrast-enhanced CT scan was acquired with a 2-mm slice thickness. Three series of CT scans were acquired in deep respiration, expiration, and free breathing. Before acquiring the CT scan, the patient was trained to breathe slowly. Intravenous contrast was just used for free-breathing series. We acquired T2 and T1 dynamic contrast-enhanced MRI images of the liver in the treatment position. All the acquired images were registered based on the fusion of the vertebral body on a Monaco treatment planning system (Elekta, AB, Stockholm, Sweden). Surgical clips, which had been placed on the tumor bed in the previous surgery, were used as fiducial markers.
2.2. Delineation of Targets and OAR
A free-breathing CT scan with IV contrast was used for radiotherapy planning and all targets and organs at risk were delineated. Gross tumor volume (GTV) was delineated on the contrast-enhanced CT scan in free breathing. It consisted of contrast-enhanced tumoral tissue and the previous surgical cavity. To create ITV, the displacement of all clips in all directions was measured in deep respiration and expiration CT series. The maximum displacement of each marker in all directions was added to GTV. Planning target volume (PTV) was made by the addition of a 5-mm margin to ITV. The stomach, liver, kidneys, lungs, heart, small intestine, duodenum, spinal cord, common bile duct, portal vein, and skin were contoured as organs at risk.
2.3. Treatment Planning
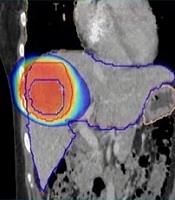
The treatment plan was generated with Monaco TPS using 11 non-coplanar conformal fields of 10 MV photon beams to prescribe 50 Gy in five fractions to 99% of GTV. The shape of each beam was defined automatically considering a 5 mm margin to PTV. We tried to achieve the targeted doses listed in Table 1 by changing gantry, table, and collimator angles and using the wedge compensator or field-in-field techniques. Attention was paid to reduce the beam entrance and exit overlap on the patient’s skin. Dose calculation was done using the Monte Carlo algorithm X-ray Voxel MC (XVMC) with a grid resolution of 2 mm. A total of 50 Gy was delivered in five fractions over 10 days in an every-other-day fashion. The treatment plan was rendered in a way that 50 Gy isodose covered 99% of the GTV outlined from CT acquired in the venous phase. The outlined structure volumes and their dose-volume relations are listed in Table 1. The ratio of the volume of the 50% isodose line to PTV was 2.628 and the plan Heterogeneity Index (HI) was 1.08. The dose distribution is shown in Figure 1.
Dose Values of Target Volumes and Organs at Risk
| Organ | Delivered Dose Values | Threshold Dose/Maximum Point Dose | Reference |
|---|---|---|---|
| GTV | V98% = 100% | N/A | N/A |
| V95% = 100% | |||
| V105% = 0% | |||
| D2cc = 51.7 Gy | |||
| ITV | V98% = 98.3% | N/A | N/A |
| V95% = 99.9% | |||
| V105% = 0% | |||
| D2cc = 52.1 Gy | |||
| PTV | V98% = 86% | N/A | N/A |
| V95% = 97% | |||
| V105% = 0% | |||
| Dmin = 50.4 Gy | |||
| D2cc = 52 Gy | |||
| Liver-GTV (normal liver tissue) | Dmean = 19 Gy | Dmean < 21 Gy | AAPM Task Group 101 |
| V21Gy = 401cc | V21Gy < 700cc | ||
| V15Gy = 576cc | |||
| Right lung | Dmean = 4 Gy | V12.5Gy < 1500cc | AAPM Task Group 101 |
| V12.5Gy = 72cc | V13.5Gy < 1000cc | ||
| Stomach | Max Point Dose = 13.6 Gy | Max Point Dose < 32 Gy | AAPM Task Group 101 |
| V18Gy = 0cc | V18Gy < 10cc | ||
| D1cc = 10.7 Gy | |||
| D5cc = 7.5 Gy | |||
| L kidney | Max Point Dose = 11 Gy | V17.5Gy < 200cc | AAPM Task Group 101 |
| Dmean = 1.2 Gy | |||
| V17.5Gy = 0cc | |||
| R kidney | Max Point Dose = 11 Gy | V17.5Gy < 200cc | AAPM Task Group 101 |
| Dmean =2.4 Gy | |||
| V17.5Gy = 0cc | |||
| Duodenum | V18Gy = 0cc | Dmax < 32Gy | AAPM Task Group 101 |
| V12.5Gy = 0cc | V18Gy < 5cc | ||
| Max Point Dose = 2.5 Gy | V12.5Gy < 10cc | ||
| Common bile duct | Dmax = 38 Gy | Dmax < 50 Gy | SPARC Protocol |
| Dmean = 10 Gy | |||
| D1cc = 18.4 Gy | |||
| Skin | Max Point Dose = 37.5 Gy | V36.5Gy < 10cc | AAPM Task Group 101 |
| V36.5Gy = 0cc | Max Point Dose < 39.5 Gy | ||
| D1cc = 33 Gy | |||
| D5cc = 28 Gy | |||
| Spinal cord | Max Point Dose =13 Gy | Dmax < 30 Gy | AAPM Task Group 101 |
| V14.5Gy = 0cc | V14.5Gy < 1.2cc | ||
| V23Gy = 0cc | V23Gy < 0.35cc | ||
| D1cc = 12.7 Gy | |||
| D5cc = 12.6 Gy |
Axial, sagittal, and coronal CT images demonstrating dose coverage of the target volume and DVH showing the dose volumes received by organs at risk. Isodose curves; the dose is shown in color-wash ranging from 50% (25 Gy) to 105% (55 Gy) of the prescription dose.

2.4. Treatment Delivery
Treatment delivery was done using Infinity (Elekta AB, Stockholm, Sweden) equipped with cone-beam CT (CBCT). A kV CT scan during the shallow breathing cycle was taken before each treatment session and setup corrections were made in XVI software based on matching the fiducial markers at the tumor bed. Radiation was delivered in free-breathing as indicated by SBRT ITV method (6) protocols as previously described. The patient tolerated the treatment uneventfully.
2.5. Dose Distribution
The delivered dose to the target volumes and relevant organs at risk are listed in Table 1. The corresponding dose constraints and recommendations excerpted from several credible guidelines are also described.
The CBCT images acquired before each treatment session was imported in the TPS and image matching was done based on the correction values in each treatment session.
Major organs at risk and the liver were delineated in each treatment session and the dose to the whole liver was calculated. The mean dose delivered to the liver in the five sessions was 21.2 ± 0.1 Gy.
2.6. Treatment Outcome
Despite a transient increase in the transaminases serum level, the patient could complete the treatment course in 10 days without experiencing any serious adverse effect. In a follow-up visit, two weeks after the treatment, she was not experiencing any discomfort or evidence of disease. Imaging confirmed stable lesions of the liver, with decreased enhancement after contrast injection. The metastatic lesions of the liver remained progression-free twelve months post-treatment when she encountered a pelvic recurrence and was put on systemic chemotherapy.
3. Discussion
Since the emergence of modern radiotherapy techniques, radiation oncologists have been able to reduce normal tissue injury, while targeting the tumor properly. Stereotactic body radiation therapy has been associated with remarkable results in various studies, with local control rates reaching up to 94% (9, 10). Stereotactic body radiation therapy was first used in medically inoperable patients and showed promising results. Evans et al. reported a case of upper urinary tract urothelial carcinoma successfully treated with 50 Gy in four fractions (11).
As the local treatment for early-stage non-small cell lung cancer, SBRT has gained increasing interest in recent years. In an RTOG phase II study of 55 patients with early-stage non-small cell lung cancer who were medically inoperable, Timmerman et al. reported a high local control rate and an OS of 58.3% in three years, compared to the 20% - 30% OS in the group of patients who underwent conventional treatment, according to the data from previous studies (12).
Delivering high energies in the range of 5 to 25 Gy in one fraction needs immaculate considerations. RTOG 0438 provides a comprehensive guideline on requirements and eligibility checklists that must be met before an institution can utilize highly conformal radiation therapy for patients with liver metastases (13). As the first administration of SBRT in Iran, we established a comprehensive plan of action that regulated each step of the process.
According to the literature, radiation-induced liver disease (RILD) due to SBRT mostly includes grade I to II toxicity and the procedure is generally well-tolerated (14). As expected, our patient experienced a transient increase in liver enzymes but suffered no permanent injury or any clinical symptoms. This study suggested SBRT as a feasible and attainable modality to treat solitary metastatic lesions when used appropriately.
References
-
1.
Timmerman RD, Kavanagh BD, Cho LC, Papiez L, Xing L. Stereotactic body radiation therapy in multiple organ sites. J Clin Oncol. 2007;25(8):947-52. [PubMed ID: 17350943]. https://doi.org/10.1200/JCO.2006.09.7469.
-
2.
Katz AW, Carey-Sampson M, Muhs AG, Milano MT, Schell MC, Okunieff P. Hypofractionated stereotactic body radiation therapy (SBRT) for limited hepatic metastases. Int J Radiat Oncol Biol Phys. 2007;67(3):793-8. [PubMed ID: 17197128]. https://doi.org/10.1016/j.ijrobp.2006.10.025.
-
3.
Benedict SH, Yenice KM, Followill D, Galvin JM, Hinson W, Kavanagh B, et al. Stereotactic body radiation therapy: The report of AAPM Task Group 101. Med Phys. 2010;37(8):4078-101. [PubMed ID: 20879569]. https://doi.org/10.1118/1.3438081.
-
4.
Solberg TD, Balter JM, Benedict SH, Fraass BA, Kavanagh B, Miyamoto C, et al. Quality and safety considerations in stereotactic radiosurgery and stereotactic body radiation therapy: Executive summary. Pract Radiat Oncol. 2012;2(1):2-9. [PubMed ID: 25740120]. [PubMed Central ID: PMC3808746]. https://doi.org/10.1016/j.prro.2011.06.014.
-
5.
Van den Begin R, Engels B, Boussaer M, Dhont J, Burghelea M, Depuydt T, et al. Motion management during SBRT for oligometastatic cancer: Results of a prospective phase II trial. Radiother Oncol. 2016;119(3):519-24. [PubMed ID: 27179921]. https://doi.org/10.1016/j.radonc.2016.04.020.
-
6.
Yang W, Fraass BA, Reznik R, Nissen N, Lo S, Jamil LH, et al. Adequacy of inhale/exhale breathhold CT based ITV margins and image-guided registration for free-breathing pancreas and liver SBRT. Radiat Oncol. 2014;9:11. [PubMed ID: 24401365]. [PubMed Central ID: PMC3896695]. https://doi.org/10.1186/1748-717X-9-11.
-
7.
Bentzen SM, Overgaard J, Thames HD, Overgaard M, Vejby Hansen P, von der Maase H, et al. Clinical radiobiology of malignant melanoma. Radiother Oncol. 1989;16(3):169-82. [PubMed ID: 2587808]. https://doi.org/10.1016/0167-8140(89)90017-0.
-
8.
Hanna GG, Murray L, Patel R, Jain S, Aitken KL, Franks KN, et al. UK consensus on normal tissue dose constraints for stereotactic radiotherapy. Clin Oncol (R Coll Radiol). 2018;30(1):5-14. [PubMed ID: 29033164]. https://doi.org/10.1016/j.clon.2017.09.007.
-
9.
Macdermed DM, Weichselbaum RR, Salama JK. A rationale for the targeted treatment of oligometastases with radiotherapy. J Surg Oncol. 2008;98(3):202-6. [PubMed ID: 18618604]. https://doi.org/10.1002/jso.21102.
-
10.
Chang BK, Timmerman RD. Stereotactic body radiation therapy: A comprehensive review. Am J Clin Oncol. 2007;30(6):637-44. [PubMed ID: 18091059]. https://doi.org/10.1097/COC.0b013e3180ca7cb1.
-
11.
Evans JD, Hansen CC, Tollefson MK, Hallemeier CL. Stereotactic body radiation therapy for medically inoperable, clinically localized, urothelial carcinoma of the renal pelvis: A case report. Adv Radiat Oncol. 2018;3(1):57-61. [PubMed ID: 29556581]. [PubMed Central ID: PMC5856982]. https://doi.org/10.1016/j.adro.2017.08.012.
-
12.
Timmerman R, Paulus R, Galvin J, Michalski J, Straube W, Bradley J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303(11):1070-6. [PubMed ID: 20233825]. [PubMed Central ID: PMC2907644]. https://doi.org/10.1001/jama.2010.261.
-
13.
Katz AW, Winter KA, Dawson LA, Schell MC, Kim JHJ, Chen Y, et al. RTOG 0438: A phase I trial of highly conformal radiation therapy for patients with liver metastases. J Clin Oncol. 2012;30(4_suppl):257. https://doi.org/10.1200/jco.2012.30.4_suppl.257.
-
14.
Pan CC, Kavanagh BD, Dawson LA, Li XA, Das SK, Miften M, et al. Radiation-associated liver injury. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S94-100. [PubMed ID: 20171524]. [PubMed Central ID: PMC4388033]. https://doi.org/10.1016/j.ijrobp.2009.06.092.