Abstract
Background:
Acquired immunodeficiency syndrome is a behavioral disorder that can be detected via two methods, including active and passive screening.Objectives:
This study aimed to evaluate the cost-effectiveness of screening strategies of HIV/AIDS among injection drug users (IDUs) referring to the voluntary counseling and testing (VCT) center and drop-in center (DIC) of Shiraz University of Medical Sciences.Methods:
This was a cross-sectional cost-effectiveness analysis to compare the cost-effectiveness of the two active and passive screening methods in 2015. The decision tree model, along with the TreeAge11 software, was used to analyze the data.Results:
The averages of cost and effectiveness were $989 and 987 subjects in the active screening method while they were $1,767 and 209 subjects in the passive screening method, respectively. The incremental cost-effectiveness ratio (ICER) to early-diagnosed and averted cases was $855/39 for the active screening method and $1528/90 for the passive screening method. According to the findings of the study, the active screening method is more cost-effective than its passive counterpart.Conclusions:
According to the findings of the study, the active screening method is more cost-effective than its passive counterpart, and it is recommended to be used in these cases.Keywords
Acquired Immunodeficiency Syndrome (AIDS) Active Screening Passive Screening Cost-Effectiveness Analysis
1. Background
Human immunodeficiency virus (HIV) infection and acquired immunodeficiency syndrome (AIDS) have become a global epidemic and one of the most important challenges to the health system over the past two decades (1). Acquired immunodeficiency syndrome is caused by the immunodeficiency virus (2) and is a type of behavioral disorder that is transmitted due to the lack of health awareness and social misconduct (3). Moreover, long-term asymptomatic HIV-positive patients are considered a potential risk (4). According to statistics, more than 35.3 million people worldwide are infected with AIDS (5). In the countries of the Eastern Mediterranean, the prevalence of HIV/AIDS is estimated at 0.2% (6). What adds to the importance of preventing and screening this disease is that there is no definitive treatment for it (7) and that people with HIV infection may remain asymptomatic for a remarkably long period and can easily transmit the infection (8). During recent years, there has been little concern about people's awareness of the transmission and prevention of the disease and dealing with affected people, and the increased risk of the disease has classified the issue as one of the health priorities (9).
Various reports have shown that most infected people in Iran are injection drug users (IDUs). However, various studies have reported a relatively different percentage in different years, ranging from 51 to 85% (10-14). Notwithstanding, what is certain is that injection drug use by a great number of people makes AIDS spread day by day (15). This image has led to effective practical measures to be taken to reduce the problem of injection drug use in the last few years; however, it seems that paying attention to this high-risk group has created the belief that other high-risk groups do not have much priority (16).
According to the study conducted by the World Health Organization (WHO), it is estimated that the number of people living with HIV in Iran is 80,000, while only about one-quarter of them have been identified (8). In the industrialized countries, the cost of medical services provided to an AIDS patient is estimated at $25-150 thousand, which is not available in developing countries (17). On the other hand, the Gross Domestic Product (GDP) of most countries has declined by the lack of human capital (16, 18) because without proper nutrition, health, and medicine, many people die due to the complications of AIDS. Moreover, since people in economically active age groups are most likely to suffer from the disease, the taxable population is thus reduced on top of available resources for public expenditure (19). At the household level, AIDS also affects the level of household income, which also increases healthcare costs. A study in Ivory Coast has shown that the cost of treatment for families with HIV-infected members is twice that of other families. These additional costs also lead to cuts in education expenditure, as well as other personal and family investments (20). Also, in reviewing the economic burden of AIDS in China in 2007, the average economic costs of AIDS prevention centers were estimated at $41.51. In this regard, given the burden of the disease, finding affected patients can be an effective step towards reducing the excessive costs of AIDS (21).
Effective screening for HIV-related cases is necessary since it can directly have extensive effects on the individual and others in the community due to the high externality associated with it (12). Besides, AIDS is a threat to development, security, and economic growth; accordingly, as reported in Sharifi’s study, each 1% increase in HIV mortality resulted in a 1.6% decline in Iran's GDP in the years 1990-2009 (22).
In Iran, the insufficient number of economic studies on HIV is evident to find community-based and cost-effective programs (23). Given the lack of financial resources in health systems in developing countries, cost-effectiveness analysis of both active and passive screening methods can help clarify the economic outcomes of these methods and also helps the policymakers and program managers to make informed decisions about resource allocation, thus allowing them to choose the most effective and efficient method by considering available resources through reviewing alternatives to achieve their goals. The existence of IDUs as the most vulnerable group to the disease in the country and the responsiveness of authorities to the development of HIV-related centers are convincing scientific and documentary evidence to justify and evaluate the costs and effectiveness of these centers.
2. Objectives
Since one of the problems of the healthcare system in Iran, like many other countries, is the high cost of treating this disease, this study aimed to determine the cost-effectiveness of active versus passive screening of cases with HIV/AIDS among IDUs referring to the voluntary counseling and testing (VCT) center using cost-effectiveness analysis in 2015.
3. Methods
This descriptive-analytical, retrospective study was implemented to evaluate the cost-effectiveness of active versus passive screening of HIV/AIDS cases among IDUs referring to the VCT center and DIC in 2015. The target population of the study included all IDUs referring to the VCT center (for passive screening) and IDUs with HIV in Fars Province (for active screening). As the active screening method is not implemented in the country, first, by using estimates from the Health Modeling Research Center and the Regional HIV Education Center, the prevalence of HIV/AIDS among IDUs in Fars Province was reported as 13.8%. Then, based on the number of IDUs estimated in Fars Province, as well as the province's population (4,592,000 people) in 2015 (24), the final number of HIV/AIDS IDUs in Fars Province was estimated at 2,845 to determine the active screening method, of whom 593 people were selected by the census method to determine the volume of the target sample in the passive screening method. The inclusion criteria included all people at risk of AIDS, as well as those who were diagnosed with the disease through using either the active or passive screening methods. Required data for this research were collected in two parts of cost and effectiveness indicators.
3.1. Cost Data
Considering that the criterion for calculating costs in this study was the provider’s perspective, and concerning the cost data extracted from documents and reports (for the passive screening method), as well as predicted costs (for the active screening method), the costs were generally classified into two categories of direct and indirect costs. To complete the costing checklist of patients for the passive screening method, 593 cases of HIV-infected IDUs referring to the VCT were used. Moreover, an expert panel was used to determine the costs of the active screening method. Accordingly, five people were selected as experts with more than five years of experience in this field and estimated the cost of the active screening method through interviewing and group meetings. Also, given that the statistics on the HIV-infected patients collected from the VCT in Shiraz belonged to the years 2004 to 2014, the following formula provided by the Central Bank was used to convert the costs to the current value) One dollar = 32,375 Rials) (the year 2015):
Cost amount *(price index number in the year 2014/price index number in the year of occurrence of cost) = Current value of costs in the year 2015
3.2. Effectiveness
To measure the effectiveness in both active and passive screening methods, the averted case index (the number of individuals not affected by the disease as a result of early diagnosis), as well as HIV-positive patients, was diagnosed at the early stages of HIV infection.
After drawing the decision tree, an incremental cost-effectiveness ratio (ICER) was calculated using the following equation. In this formula, the cost refers to the average cost (US $), and the effectiveness indicates the individual effectiveness according to the mentioned indices.
Incremental Cost-effectiveness Ratio (ICER) = ∆C/(∆E) = C1-C0/E1-E0
To perform the cost-effectiveness analysis, TreeAge version 2011 software was used.
3.3. Sensitivity Analysis
To cope with uncertainty, one-way deterministic sensitivity analysis (Tornado Diagram) and probabilistic sensitivity analysis were carried out. In the one-way sensitivity analysis, the value of each parameter was increased by 20%, and the results were presented by the Tornado diagram.
4. Results
The average direct costs per capita estimated in both active and passive screening methods were $803.69 and $1337.21, respectively (Table 1).
Direct Costs of the Active and Passive Screening Methods for HIV/AIDS-Positive Injection Drug Users
| Items | Active Screening Method | Passive Screening Method | ||
|---|---|---|---|---|
| Per Active Screening ($US) | Total Active Costs (Number of Patients to be Covered by the Active Screening Method, N = 2845) | Per Passive Screening ($US) | Total Cost of Inactive Files ($US) (N = 593) | |
| Medicine | 254.94 | 725285. 34 | 254. 94 | 151200 |
| Counseling Services | 37.34 | 106213. 34 | 37. 34 | 22133. 34 |
| Hospital Services | 144 | 409680 | 144 | 85333. 34 |
| Personnel wages and salary | ||||
| Physicians and specialists | 93.73 | 266666. 67 | 269. 34 | 160000 |
| Other staff | 124.10 | 353066. 67 | 520 | 306666. 67 |
| Healthcare services (physician visit) | 88 | 250360 | 88 | 52000 |
| Costs of laboratory tests | 42. 67 | 121386. 67 | 42. 67 | 25333. 34 |
| Prevention services (syringes, condoms, etc.) | 18. 94 | 53865. 34 | 18. 94 | 11200 |
| Total | 803. 69 | 2286521. 6 | 1375.2 | 813866. 67 |
The results indicated that the average indirect costs per capita in both active and passive screening methods were $16.456 and $156.54, respectively (Table 2).
Indirect Costs of Active and Passive Screening Methods for HIV/AIDS-Positive Injection Drug Users
| Items | Active Screening Method | Passive Screening Method | ||
|---|---|---|---|---|
| Per Active Screening (US $) | Total Active Costs (Number of Patients to be Covered by the Active Screening Method, N = 2845) | Per Passive Screening ($US) | Total Cost of Inactive Files ($US) | |
| Education | 8 | 19194.26 | 6.74 | 4000 |
| Depreciation of medical equipment | 0.62 | 373.34 | 0.62 | 373.34 |
| Depreciation of building | 48/40 | 24000 | 40.48 | 24000 |
| Depreciation of transportation vehicles | 11.2 | 31866.87 | 2.24 | 1333.34 |
| Depreciation of laboratory equipment | 3.82 | 10848.94 | 3.81 | 2266.67 |
| Mobile medical team | 72 | 42666.67 | 72 | 42666.67 |
| Energy costs | 8.10 | 4800 | 8.10 | 4800 |
| Costs related to equipment for recording information | 22.48 | 13333.34 | 22.48 | 13333.34 |
| Total | 165.456 | 147083.2 | 156.54 | 92773.34 |
According to Table 3, the total direct and indirect costs in both active and passive screening methods were $855.39 and $1528.90, respectively (Table 3).
Costs of Active and Passive Screening Methods for HIV/AIDS-positive Injection Drug Users
| Costs | Active Screening Method | Passive Screening Method |
|---|---|---|
| Direct costs ($US) | 2286524 | 813866.67 |
| Indirect costs ($US) | 147083.2 | 92773.34 |
| Total costs ($US) | 2433607.2 | 906640 |
| Mean values of total active screening costs per person ($US) | 855.39 | 1528.90 |
In this study, a decision tree model was applied to estimate the expected cost and effectiveness of passive and active screening methods (Figure 1). In Figure 1, the terminal nodes of the tree represent the average cost, and the values under the branch indicate probabilities.
Decision trees for the active and passive screening methods estimated for the HIV/AIDS-positive IDUs referred to the VCT center affiliated to Shiraz University of Medical Sciences (in 2015). Translation: Active/passive positive result of ELISA Test; Negative result of ELISA Test; Positive result of the second ELISA Test; Negative result of the second ELISA Test
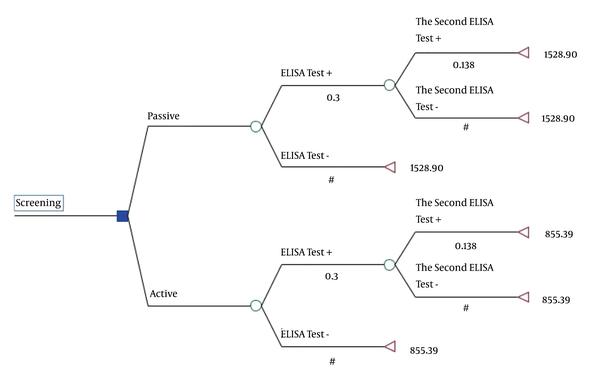
According to the results shown in Table 4, the expected effectiveness and cost were calculated to be 987 and $855.39 for active screening and 209 and $1,528.90 for passive screening, respectively. Therefore, active screening is dominant compared to passive screening (less costly and more effective), and there was no need to calculate an ICER. The total cost-saving of active screening compared to passive screening was $523,990 (Table 4).
Estimated Incremental Cost-effectiveness Ratio of Active Versus Passive Screening Method for HIV/AIDS-positive Injection Drug Users Referring to the Voluntary Counseling and Testing Center Affiliated to Shiraz University of Medical Sciences in 2015
| Screening method | Expected Costs ($US) | Expected Effectiveness | Cost Difference ($US) | Effectiveness Difference | Active vs. Passive | Total Cost Savings ($US) |
|---|---|---|---|---|---|---|
| Active | 855.39 | 987 | -673.51 | 778 | Dominant | (778*673. 51) 523990.78 |
| Passive | 1528.90 | 209 |
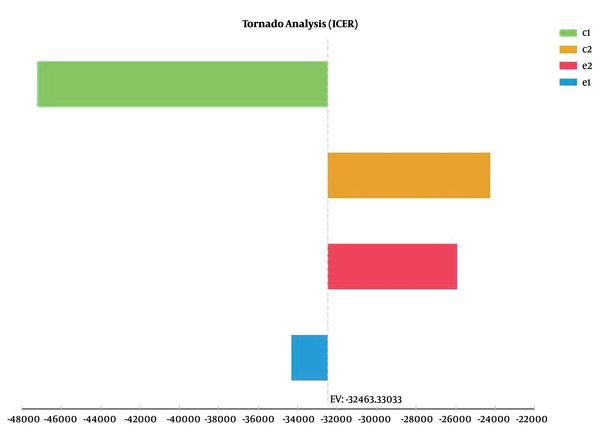
The Tornado diagram in Figure 2 shows that the results of the study had the highest and lowest sensitivity to the cost and effectiveness of the passive screening method, respectively.
Tornado analysis to investigate the sensitivity of the active versus passive screening methods for the HIV/AIDS positive IDUs referring to the VCT center affiliated to Shiraz University of Medical Sciences (in 2015). * In the above figure, c1 and e1 represent the cost and effectiveness of the passive screening method, and c2 and e2 stand for the cost and effectiveness of the active screening method, respectively.
The results of probabilistic sensitivity analysis using Monte Carlo simulation are indicated in Figure 3. The results showed that active screening was more cost-effective than passive screening, with a maximum willingness-to-pay threshold of $49,521 calculated based on the WHO method (three times of per capita GDP, $49,521).
The willingness-to-pay chart of the active versus passive screening methods for HIV/AIDS-positive IDUs referring to the VCT center affiliated to Shiraz University of Medical Sciences (in 2015)
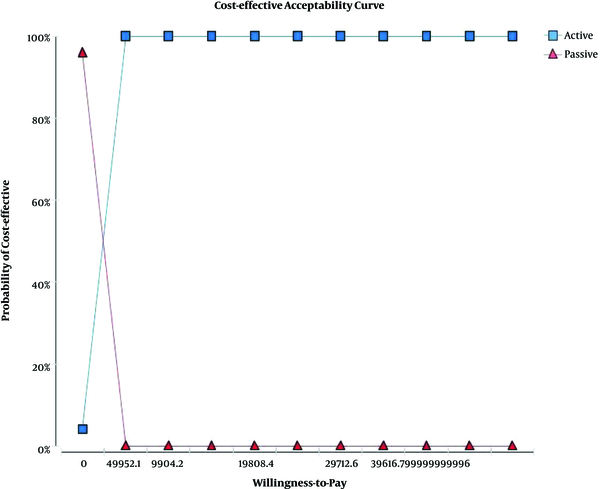
Figure 3 indicates the cost-effectiveness acceptability curve (CEAC) of the active versus passive screening methods. The horizontal axis shows the amount of willingness-to-pay (in dollars), and the vertical axis shows the probability of the cost-effectiveness of the active screening method compared to the passive one. According to the chart, in the willingness-to-pay amount of $2,476, the cost-effectiveness probability of active and passive screening was equal to each other (roughly 50%), and after this rate, the cost-effective probability of the active screening method was about 96% compared to passive screening.
5. Discussion
According to the results of the present study, both active and passive screening methods had the highest direct costs. Of direct costs associated with the active and passive screening methods, the highest costs were allocated to medicine and personnel wages, representing 31% and 57% of the total costs in both active and passive screening methods, respectively. On the other hand, the lowest direct costs in both active and passive screening methods were related to the prevention services. Direct costs measure the opportunity costs of the resources used in the treatment of a particular disease; however, indirect costs measure the value of missed resources in diseases (25).
As can be inferred from the findings of this study, of indirect costs associated with the active and passive screening methods, the highest cost was associated with the mobile medical team, consisting of 29% and 45% of the total indirect costs in active and passive screening methods, respectively. Also, the lowest indirect cost was related to the depreciation of medical equipment in both active and passive screening methods. Hesam et al. (2014) also found that building maintenance costs (equal to $972.42) were the highest in capital expenditures. On the other hand, the personnel wage amounting to $35,840 had the highest rank among the current costs of the private and public VCT centers under the supervision of Shiraz University of Medical Sciences and Health Services (24). The results of a study conducted by Keshtkaran et al. (2012) examining the cost-effectiveness of methadone maintenance treatment centers for the prevention of HIV among IDUs indicated that the personnel wages, as part of the current costs, had the highest value and amounted to $287,626.13. However, transportation costs as a fraction of the current costs had the lowest value (about $197.38) (26).
According to the findings of the present study, the mean value of the total direct and indirect costs ($1528.90) in the passive screening method was greater than the estimated value ($855.39) in the active screening method. Keshtkaran et al. (2012) showed that the intervention costs (center spending) excluding non-IDUs were $204,297.7. In their study, the non-intervention cost was estimated to be $13,942,756.8 (26).
The results of this study showed that the active screening method was more cost-effective than the passive screening method concerning the averted case index. Therefore, the passive screening method is not an appropriate option since it is costlier and less effective. On the other hand, the active screening method is a cost-effective option and is recommended for the treatment of HIV-positive IDUs. This could be due to the high costs of human resources (personnel salary and wages) in the passive screening method compared to those in active screening. Hence, given that the effectiveness index calculated in the active screening method was greater than that of the passive screening method and its cost. In addition, the results of the one-way and probabilistic sensitivity analysis confirmed that the active screening method was more cost-effective than the passive screening method.
Vankates et al. (2013) showed that early screening was associated with the faster diagnosis of high-risk groups. On the other hand, early screening also increases the average survival time among the affected population. The results of a study in India also revealed that the voluntary HIV screening every three to five years for the general population, excluding low-risk groups, was justified regarding clinical and cost-effectiveness aspects. In this regard, the early screening of the general population may also be affordable (27). Yazdan Panah et al. (2010) concluded that one-time screening in France compared to the common screening method and other proposed screening interventions proposed in Western Europe led to an increase in the patients' survival rate (28). In addition, Paltil et al. (2005) found that early HIV screening was associated with a faster diagnosis of diseases of high-risk populations, and the early screening also increased the average survival time in the affected population. Similarly, they claimed that the voluntary HIV screening every three to five years for the general population, excluding low-risk groups, is justified regarding the clinical and cost-effectiveness aspects. The early screening of the general population may also be affordable in this regard (29). Sanders et al. (2005) showed that the cost-effectiveness of the routine HIV screening in healthcare centers, even in populations with a relatively low HIV prevalence, is similar to common interventions; thus, such programs should be developed (30). In addition, we can generalize these results to other Iranian settings. However, we cannot generalize these results to other countries certainly due to differences in the patients’ ability to pay, the incidence and prevalence of HIV, differences in clinical guidelines, relative prices, payment systems, and ceiling ratios.
5.1. Conclusion
Given that active screening is dominant compared to passive screening, it is suggested that active screening be used for the early identification of HIV in injection drug users.
Acknowledgements
References
-
1.
Hasanshahi M, Baghbanian A, Motazedian N. Awareness, Attitudes and Tendency Toward Providing Services to Patients With HIV/AIDS by Second- and Third-Year Nursing Students in Isfahan, Iran, 2014. Womens Health Bull. 2016;4(2). https://doi.org/10.17795/whb-32339.
-
2.
Khani Jeihooni A, Arameshfard S, Hatami M, Mansourian M, Kashfi SH, Rastegarimehr B, et al. The effect of educational program based on health belief model about HIV/AIDS among high school students. Int J Pediatr. 2018;6(3):7285-96.
-
3.
Ayranci U. AIDS knowledge and attitudes in a Turkish population: an epidemiological study. BMC Public Health. 2005;5:95. [PubMed ID: 16159400]. [PubMed Central ID: PMC1242238]. https://doi.org/10.1186/1471-2458-5-95.
-
4.
Shokuhi S, Gachkar L, Alavi-Darazam I, Yuhanaee P, Sajadi M. Occupational Exposure to Blood and Body Fluids among Health Care Workers in Teaching Hospitals in Tehran, Iran. Iran Red Crescent Med J. 2012;14(7):402-7. [PubMed ID: 22997555]. [PubMed Central ID: PMC3438432].
-
5.
Rosen S, Fox MP. Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review. PLoS Med. 2011;8(7). e1001056. [PubMed ID: 21811403]. [PubMed Central ID: PMC3139665]. https://doi.org/10.1371/journal.pmed.1001056.
-
6.
Avanessian E, Naserirad M, Abrahamian H, Anis S. Size of Social Network and Probability of Occurrence of HIV/AIDS among Sexually Affected Patients in Behavioral Diseases Consulting Centers. J Health Educ Health Promot. 2017;5(2):73-80. https://doi.org/10.30699/ihepsa.journal.5.2.1.
-
7.
Idele P, Gillespie A, Porth T, Suzuki C, Mahy M, Kasedde S, et al. Epidemiology of HIV and AIDS among adolescents: current status, inequities, and data gaps. J Acquir Immune Defic Syndr. 2014;66 Suppl 2:S144-53. [PubMed ID: 24918590]. https://doi.org/10.1097/QAI.0000000000000176.
-
8.
Khani-Jeihouni A, Ranjbari S, Khiyali Z, Moradi Z, Motamedi MJ. Evaluation of the Factors Associated with AIDS Prevention Performance among Male Barbers Based on the Health Belief Model in Fasa. J Educ Community Health. 2017;3(4):59-65. https://doi.org/10.21859/jech.3.4.59.
-
9.
Kanda K, Obayashi Y, Jayasinghe A, Silva KT, Lee RB, Tamashiro H. Current HIV/AIDS knowledge, perceptions and practices among the general population in Kandy, Sri Lanka: program implications. J Int Health. 2010;25(1):11-9.
-
10.
Stover J, Rosen J, Kasedde S, Idele P, McClure C. The impact and cost of the HIV/AIDS investment framework for adolescents. J Acquir Immune Defic Syndr. 2014;66 Suppl 2:S170-5. [PubMed ID: 24918592]. https://doi.org/10.1097/QAI.0000000000000174.
-
11.
Zamani S, Kihara M, Gouya MM, Vazirian M, Ono-Kihara M, Razzaghi EM, et al. Prevalence of and factors associated with HIV-1 infection among drug users visiting treatment centers in Tehran, Iran. AIDS. 2005;19(7):709-16. [PubMed ID: 15821397]. https://doi.org/10.1097/01.aids.0000166094.24069.72.
-
12.
Vazirian M, Nassirimanesh B, Zamani S, Ono-Kihara M, Kihara M, Ravari SM, et al. Needle and syringe sharing practices of injecting drug users participating in an outreach HIV prevention program in Tehran, Iran: a cross-sectional study. Harm Reduct J. 2005;2:19. [PubMed ID: 16212655]. [PubMed Central ID: PMC1266391]. https://doi.org/10.1186/1477-7517-2-19.
-
13.
Khajehkazemi R, Osooli M, Sajadi L, Karamouzian M, Sedaghat A, Fahimfar N, et al. HIV prevalence and risk behaviours among people who inject drugs in Iran: the 2010 National Surveillance Survey. Sex Transm Infect. 2013;89 Suppl 3:iii29-32. [PubMed ID: 24037249]. [PubMed Central ID: PMC3841768]. https://doi.org/10.1136/sextrans-2013-051204.
-
14.
Asadi H, Imani-Nasab M, Garavand A, Hasoumi M, Kia AA, Haghi B, et al. HIV Positive Patients' Experience of Receiving Health Care Services: A Phenomenology Study in Iran. Open AIDS J. 2018;12(1):150-61. https://doi.org/10.2174/1874613601812010150.
-
15.
Masoudnia E. Public perceptions about HIV/AIDS and discriminatory attitudes toward people living with acquired immunodeficiency syndrome in Iran. SAHARA J. 2015;12:116-22. [PubMed ID: 26726933]. https://doi.org/10.1080/17290376.2015.1123644.
-
16.
Negin J, Barnighausen T, Lundgren JD, Mills EJ. Aging with HIV in Africa: the challenges of living longer. AIDS. 2012;26 Suppl 1:S1-5. [PubMed ID: 22713477]. [PubMed Central ID: PMC4017661]. https://doi.org/10.1097/QAD.0b013e3283560f54.
-
17.
Mogarehi M, Shokranian N. Knowledge and attitude of student nurses towards AIDS. Iran J Nurs. 2003;16(34):19-24.
-
18.
Bell C, Devarajan S, Gersbach H. The long-run economic costs of AIDS: theory and an application to South Africa. 2003. https://doi.org/10.1596/1813-9450-3152.
-
19.
Pulver S. Organising Business. Greener Manag Int. 2002;2002(39):55-67. https://doi.org/10.9774/GLEAF.3062.2002.au.00007.
-
20.
Dadipoor S, Ghaffari M, Safari-Moradabadi A. University students and AIDS: a systematic review of knowledge, attitudes towards AIDS in Iran. Int J Adolesc Youth. 2020;25(1):861-71. https://doi.org/10.1080/02673843.2020.1758173.
-
21.
Ekhtiari H, Noroozi A, Farhoudian A, Radfar SR, Hajebi A, Sefatian S, et al. The evolution of addiction treatment and harm reduction programs in Iran: a chaotic response or a synergistic diversity? Addiction. 2020;115(7):1395-403. [PubMed ID: 31737965]. https://doi.org/10.1111/add.14905.
-
22.
Sharifi RH, Akhoondi N, Honarvar N, Mohammadi M. Effects of the Human Immunodeficiency Virus (AIDS) epidemic on economic growth in Iran. J Res Health. 2014;4(3):770-7.
-
23.
Yaghoobi H, Ahmadinia H, Shabani Z, Vazirinejad R, Safari R, Shahizadeh R, et al. Life expectancy and years of life lost in HIV patients under the care of BandarAbbas Behavioral Disorders Counseling Center. Nepal J Epidemiol. 2017;7(4):702-12. [PubMed ID: 30510838]. [PubMed Central ID: PMC6204067]. https://doi.org/10.3126/nje.v7i4.20627.
-
24.
Hesam S, Honarvar N, Vahdat S. The Analysis of Cost-Effectiveness of Methadone and Buprenorphine Maintenance Treatment for Preventing HIV Infection in Drug-Injection Users (A Case Study: The Selected Withdrawal Centers under the Supervision of Shiraz University of Medical Sciences). J Health Account. 2014;3(3):18-39.
-
25.
Paxton A, Carvalho N. HIV/AIDS Program Costing Tools: Concepts and Methods Used under the USAID Health Policy Initiative, Costing Task Order. Washington, DC: Futures Group, Health Policy Initiative; 2013.
-
26.
Keshtkaran A, Heidari AR, Javanbakht M, Mirahmadizadeh A. Cost-effectiveness of methadone maintenance centers to prevent HIV among intravenous drug users. Payesh. 2012;11(6):823-30.
-
27.
Venkatesh KK, Becker JE, Kumarasamy N, Nakamura YM, Mayer KH, Losina E, et al. Clinical impact and cost-effectiveness of expanded voluntary HIV testing in India. PLoS One. 2013;8(5). e64604. [PubMed ID: 23741348]. [PubMed Central ID: PMC3669338]. https://doi.org/10.1371/journal.pone.0064604.
-
28.
Yazdanpanah Y, Sloan CE, Charlois-Ou C, Le Vu S, Semaille C, Costagliola D, et al. Routine HIV screening in France: clinical impact and cost-effectiveness. PLoS One. 2010;5(10). e13132. [PubMed ID: 20976112]. [PubMed Central ID: PMC2956760]. https://doi.org/10.1371/journal.pone.0013132.
-
29.
Paltiel AD, Weinstein MC, Kimmel AD, Seage GR, Losina E, Zhang H, et al. Expanded screening for HIV in the United States--an analysis of cost-effectiveness. N Engl J Med. 2005;352(6):586-95. [PubMed ID: 15703423]. https://doi.org/10.1056/NEJMsa042088.
-
30.
Sanders GD, Bayoumi AM, Sundaram V, Bilir SP, Neukermans CP, Rydzak CE, et al. Cost-effectiveness of screening for HIV in the era of highly active antiretroviral therapy. N Engl J Med. 2005;352(6):570-85. [PubMed ID: 15703422]. https://doi.org/10.1056/NEJMsa042657.