Abstract
Background:
Prevention of death in patients on the waiting list for liver transplantation (LT) is a major concern to prioritize organ allocation. Since the model for the end-stage liver disease (MELD) and its modifications have many shortages, there is a need for further refinement of the allocation strategy.Objectives:
The current study aimed at assessing the predictors of mortality in LT candidates in a more comprehensive manner with the possible implications to improve the care of such patients and assist in developing better strategies for organ allocation.Methods:
In the current cohort study, 544 adult LT candidates with end-stage liver disease were followed up for a mean of 12 months in three-month intervals. Data analysis was performed in Nutritionist, SPSS, and R software, using Kaplan-Meier, Cox proportional hazard (HRC), and LASSO Cox regression hazard (HRL) tests.Results:
The mean age of the patients was 46.7 ± 13.7 years; the majority were male (n = 336, 61.7%). At the end of the study, 414 (76.1%) subjects were still alive and 130 (23.9%) dead. The cumulative percentages of death were 33.1%, 57.7%, and 79.2% after 3, 6, and 12 months of waiting for a donor, respectively. Although there was a strong association between having hepatopulmonary syndrome (HPS) (HRC = 4.7, HRL = 1.8), a history of myocardial infarction (MI) (HRC = 3.3, HRL = 1.6), low-carbohydrate (CHO) diet (HRC = 2.7, HRL = 1.5), and mortality, it was weak for MELD score. Moreover, a serum level of CA 125, high polymorphonuclear (PMN) count, weight loss, a high level of alanine aminotransferase (ALT), positive hepatitis B virus (HBV) markers, high mean corpuscular volume (MCV) of red blood cells, ascites, and edema of gallbladder wall had association with mortality in LT patients.Conclusions:
In addition to MELD score, HPS, a history of MI, low CHO intake, weight loss, ascites, PMN, CA 125, ALT, hepatitis B surface antigen, MCV, blood urea nitrogen, and gallbladder wall thickness are predictors of mortality in LT candidates and need to be considered in the LT allocation system.Keywords
Survival Waiting List Liver Transplantation Cirrhosis, End-stage Liver Disease
1. Background
Disability-adjusted life years (DALYs) and deaths caused by cirrhosis and chronic liver diseases increased worldwide by 37.9% and 46% from 1990 to 2016, respectively (1, 2). As a result, demand increases for liver transplantation (LT), the most effective treatment for end-stage liver disease (ESLD) (3, 4). Moreover, the demand-supply imbalance caused a long waiting list (WL) in many centers (5, 6), resulting in the death of a significant proportion of such patients before having a chance to receive transplants (5). The Child-Turcotte-Pugh (CTP) and the model for end-stage liver disease (MELD) were introduced to predict the outcome in patients on WL of LT (7, 8); however, several studies revealed that these scores alone are insufficient, and there is a great need to consider other predictive factors of mortality in them (7, 9).
2. Objectives
The current study aimed at assessing the predictors of mortality in LT candidates in a more comprehensive manner with possible implications to improve the care of such patients and assist in developing better strategies for organ allocation.
3. Methods
3.1. Design and Sampling
The current prospective cohort study, after excluding patients not providing consent to participate in the research, was conducted on 544 adult patients with ESLD, aged ≥ 18 years. The subjects had been referred to the Shiraz Organ Transplant Center from different regions of Iran. They were registered as candidates for LT by a multidisciplinary team consisted of transplantation surgeons, hepatologists, pathologists, radiologists, and nutritionists. All subjects provided written informed consent after enrollment, while voluntary participation was respected in all stages of the study. The participants’ privacy was assured, including interview and gathering, recording, analysis, and reporting of data. The current study protocol was approved by the Ethics Committee of Shiraz University of Medical Sciences (SUMS) (ethical code: IR.SUMS.REC.1396.S1000).
3.2. Data Collection
For data gathering, a trained team, including a general practitioner (GP), a nutritionist, and two public health graduates, were recruited. The first assessment of patients was performed at Shiraz Organ Transplantation Center in patients presence, but the next follow-ups were carried out based on medical records through phone calls with patients or their first-degree relatives (in cases of dead patients). A comprehensive checklist, consisting of baseline demographic and socio-economic characteristics, patient health self-assessment, a history of cigarette or hookah smoking, alcohol drinking, drug use, diabetes mellitus (DM), hypertension (HTN), hyperlipidemia, asthma, gastrointestinal (GI) diseases, kidney diseases, and cancer, was completed for each subject. The data were taken from patients in the first visit based on self-report. Baseline findings from physical examination (PE), laboratory test results, ultrasound report of the hepatobiliary system, and cause of death (in dead cases) were extracted from the medical records of patients and transferred into the checklist by the GP. Causes of ESLD, such as hepatitis B virus (HBV) or hepatitis C virus (HCV) infection, primary sclerosing cholangitis (PSC), autoimmune hepatitis (AIH), non-alcoholic fatty liver disease (NAFLD), non-alcoholic steatohepatitis (NASH), primary biliary cirrhosis (PBC), hepatocellular carcinoma (HCC), alcoholic cirrhosis, drug-induced cirrhosis, cystic fibrosis (CF), and hemochromatosis, were also queried and extracted from patients’ medical records by the GP. The complications of ESLD, such as portal hypertensive gastropathy, spontaneous bacterial peritonitis (SBP), hepatorenal syndrome, hepatopulmonary syndrome (HPS), GI bleeding, and lethargy, were extracted from medical records and entered into the checklist by the GP. The nutritional status of each patient was also assessed by a clinical nutritionist through an interview-based 24-hour dietary recall. In addition, the fibrosis-4 (FIB-4) index was calculated as an accurate marker of liver fibrosis (10).
3.3. Data Analysis
Gathered data of nutrition were transferred into the nutritionist software and then analyzed by a nutritionist. Quality assurance of data entry was accomplished through double-checking. For survival analysis, the final event was defined as death and censored cases, included patients staying alive till the end of the study. For patients who received transplants, the duration of waiting on the LT list was considered. To assess the univariable effect of each variable on the survival of patients, the Cox proportional hazard (CPH), and for multiple variable analysis of survival, two different variable selection techniques were used. In the first modeling approach, all variables with a P-value of < 0.2 in univariable analysis were included in the CPH model, and a forward conditional method of variable selection, with an alpha-to-enter of 0.10, was used. As the second approach, all variables were transferred into the least absolute shrinkage and selection operator (LASSO) Cox regression (LCR) model. The LCR model, one of the modern variable selection techniques, performs simultaneous estimation and variable selection. It is applicable even when the number of variables is more than that of the sample size and very appropriate in high dimensional settings, while it provides more accurate estimates than CPH (11, 12). Descriptive statistics and CPH were performed in the IBM SPSS version 20, and LCR modeling was implemented in Package glmnet, using R 3.3.1 software. In the current study, no univariable analysis of three components of the MELD score was performed (creatinine, INR, and total bilirubin) since the MELD score was included as the representative of the three variables in the analysis.
4. Results
The mean age of patients was 46.7 ± 13.7 years, and the male (n = 336) to female (n = 208) ratio was 1.6. Patient follow-up duration was 5948.5 (person-month (the mean follow-up duration was 11.9 ± 10.9 and 7.8 ± 8.3 months for alive and dead patients, respectively). Table 1 shows the socio-economic and demographic characteristics of the patients. Out of 544 patients, 93 (17.1%) smoked cigarettes, and 22 (4%) hookah, 28 (5.1%) abused opioids, and 47 (8.6%) consumed alcohol. According to the patient self-reports, 93 (17.1%) had DM, 30 (5.5%) were known cases of HTN, 35 (6.4%) had hyperlipidemia, 51 (9.4%) asthma, 137 (25.2%) a kind of GI disease, 70 (12.9%) a kind of kidney disease, and 10 (1.8%) cancers, such as HCC.
Demographic and Socio-economic Characteristics of Patients on Waiting List for Liver Transplantation at Shiraz University of Medical Sciences, Shiraz, Irana
| Characteristic | Characteristic | ||
|---|---|---|---|
| Age (y) | Being the head of family | ||
| Mean ± SD | 46.7 ± 13.7 | Yes | 304 (55.8) |
| Median | 48 | No | 240 (44.1) |
| Min-max | 18-91 | Occupational status | |
| Gender | Unemployed | 302 (55.5) | |
| Male | 336 (61.7) | Employed | 161 (29.5) |
| Female | 208 (38.2) | Retired | 81 (14.8) |
| Marital status | Supplementary insurance | ||
| Married | 446 (81.9) | Yes | 238 (43.7) |
| Single | 81 (14.8) | No | 306 (56.2) |
| Divorced/widowed | 17 (3.1) | Working hours per day | |
| Education (y) | ≤ 8 | 527 (96.8) | |
| < 6 y | 269 (49.4) | > 8 | 17 (3.1) |
| 6 - 12 yr | 170 (31.2) | Self-health assessment | |
| > 12 yr | 105 (19.3) | Very bad to bad | 141 (25.9) |
| Family dimension | Moderate to very good | 403 (74.0) | |
| Mean ± SD | 4.0 ± 1.6 | ||
| Median | 4.0 | ||
| Min-max | 1 - 12 |
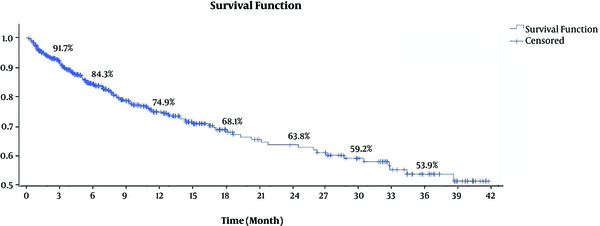
As already defined in the causes of ESLD in the medical records, 89 (16.3%) and 43 (7.9%) patients were the known cases of HBV and HCV, respectively. Also, PSC (n = 59; 10.8%), AIH (n = 44; 8%), NAFLD or NASH (n = 14; 2.5%), PBC (n = 12; 2.2%), HCC (n = 11; 2%), and alcoholic cirrhosis (n = 7; 1.2%) were among other causes. Moreover, 163 (29.9%) patients were defined as drug-induced cirrhosis, CF, or hemochromatosis. Lethargy (n = 222; 40.8%), edema (n = 151; 27.7%), and weight loss (n = 100; 18.3%) were the most prevalent symptoms in the patients. Table 2 shows the statistically significant determinants of mortality in patients on the WL for liver transplantation; variables without a significant correlation are not shown in Table 2, as well as in Tables 3 and 4, and Figure 1. The baseline MELD scores for alive and dead patients were 14.9 ± 5.7 and 18.9 ± 6.8, respectively (P < 0.001). The nutritional analysis revealed that 60.3% (60.3 ± 11.2%), 22.1% (22.1 ± 9.4%), and 17.3% (17.3 ± 5.4%) of the patient daily diet included carbohydrates (CHO), fat, and protein, respectively. The nutritional univariable analysis is shown in Table 3. At the end of the current study, 414 (76.1%) patients were alive and 130 (23.9%) dead. HPS (CPH = 4.7, LCR = 1.8), history of myocardial infarction (MI) (CPH = 3.3, LCR = 1.6), and low CHO diet (CPH = 2.7, LCR=1.5) had strong associations with patient mortality (Table 4). In addition, the one-, two-, and three-year survival rates of the patients were 74.9%, 63.8%, and 53.9%, respectively (Figure 2). It was also found that 33.1%, 57.7%, and 79.2% of total deaths occurred in the first 3, 6, and 12 months of entering the WL for LT, respectively. Three-month mortality of patients on WL was mainly associated with high PMN count (HR = 3.3), high MCV (HR = 3.1), and high MCH (HR = 2.8), while main determinants of six-month mortality were CA125 (HR = 2.5), HBsAg positivity (HR = 2.4), and high MCV (HR = 2.4) (Figure 1). In the first 12 months of follow-up, mortality was more affected by high PMN count (HR = 3.1), history of MI (HR = 3.0), and CA125 (HR = 2.8).
Univariate Analysis of Determinants of Mortality in Patients on Waiting List for Liver Transplantation at Shiraz University of Medical Sciences, Iran
| Determinant | Survived (N = 414) | Dead (N = 130) | HR | CI 95% | P-Value |
|---|---|---|---|---|---|
| Marital status | |||||
| Single | 70 (16.9) | 11 (8.4) | Baseline | ||
| Married | 331 (79.9) | 115 (88.4) | 1.94 | 1.04 - 3.60 | 0.04 |
| Divorced/widowed | 13 (3.1) | 5 (3.8) | 2.26 | 0.72 - 7.09 | 0.16 |
| Self-health assessment | |||||
| Very bad to bad | 87 (21.0) | 54 (41.5) | Baseline | ||
| Moderate to very good | 327 (78.9) | 76 (58.4) | 0.54 | 0.38 - 0.76 | 0.001 |
| Portal hypertensive gastropathy | |||||
| No | 309 (74.6) | 83 (63.8) | Baseline | ||
| Yes | 105 (25.3) | 47 (36.1) | 1.62 | 1.13 - 2.32 | 0.008 |
| Ascities | |||||
| No | 224 (54.1) | 44 (33.8) | Baseline | ||
| Yes | 190 (45.8) | 86 (66.1) | 2.05 | 1.43 - 2.95 | < 0.001 |
| Spontaneous bacterial peritonitis | |||||
| No | 393 (94.9) | 108 (83.0) | Baseline | ||
| Yes | 21 (5.0) | 22 (16.9) | 2.29 | 1.44 - 3.62 | |
| Hepatorenal syndrome | |||||
| No | 413 (99.7) | 127 (97.6) | Baseline | ||
| Yes | 1 (0.2) | 3 (2.3) | 4.21 | 1.33 - 13.27 | 0.014 |
| Hepatopulmonary syndrome | |||||
| No | 413 (99.7) | 127 (97.7) | Baseline | ||
| Yes | 1 (0.2) | 3 (2.3) | 6.74 | 2.13 - 21.33 | 0.001 |
| Myocardial infarction | |||||
| No | 414 (100) | 125 (96.1) | Baseline | ||
| Yes | 0 (0) | 5 (3.8) | 4.41 | 1.80 - 10.80 | 0.001 |
| Weight loss | |||||
| No | 350 (84.5) | 94 (72.3) | Baseline | ||
| Yes | 64 (15.4) | 36 (27.6) | 1.64 | 1.11 - 2.40 | 0.01 |
| Edema | |||||
| No | 316 (76.3) | 77 (59.2) | Baseline | ||
| Yes | 98 (23.6) | 53 (40.7) | 1.57 | 1.11 - 2.40 | 0.01 |
| Upper GI bleeding | |||||
| No | 402 (97.1) | 120 (92.3) | Baseline | ||
| Yes | 12 (2.8) | 10 (7.6) | 1.77 | 0.93 - 3.38 | 0.08 |
| Lethargy | |||||
| No | 263 (63.5) | 59 (45.3) | Baseline | ||
| Yes | 151 (36.4) | 71 (54.6) | 1.83 | 1.30 - 2.59 | 0.001 |
| Hbs Ag | |||||
| Negative | 358 (86.4) | 102 (78.4) | Baseline | ||
| Positive | 56 (13.5) | 28 (21.5) | 1.70 | 1.11 - 2.58 | 0.01 |
| Hbe Ag | |||||
| Negative | 405 (97.8) | 121 (93.0) | Baseline | ||
| Positive | 9 (2.1) | 9 (6.9) | 2.61 | 1.32 - 5.14 | 0.006 |
| Blood lymphocyte (% of WBC) | |||||
| ≥ 24 | 282 (68.1) | 73 (56.1) | Baseline | ||
| < 24 | 132 (31.8) | 57 (43.8) | 1.60 | 1.13 - 2.26 | 0.01 |
| Platelet count (x109/L) | |||||
| ≥ 150,000 | 119 (28.7) | 18 (13.8) | Baseline | ||
| < 150,000 | 295 (71.2) | 112 (86.1) | 2.04 | 1.24 - 3.35 | 0.005 |
| CA 125 (U/mL) | |||||
| ≤ 35 | 130 (31.4) | 16 (12.3) | Baseline | ||
| > 35 | 284 (68.5) | 114 (87.6) | 3.24 | 1.92-5.47 | < 0.001 |
| Gallbladder wall edema (detected by sonography) | |||||
| No | 138 (33.3) | 27 (20.7) | Baseline | ||
| Yes | 276 (66.6) | 103 (79.2) | 1.62 | 1.06 - 2.48 | 0.03 |
| Blood WBC count (x106/L) | |||||
| ≤ 10000 | 373 (90.0) | 110 (84.6) | Baseline | ||
| > 10000 | 41 (9.9) | 20 (15.3) | 1.95 | 1.21 - 3.15 | 0.006 |
| Blood PMN (% of WBC) | |||||
| ≤ 80 | 399 (96.3) | 120 (92.3) | Baseline | ||
| > 80 | 15 (3.6) | 10 (7.6) | 2.45 | 1.28 - 4.67 | 0.01 |
| Blood RBC7 count (x1012/L) | |||||
| 4.6 ≤ | 101 (24.3) | 16 (12.3) | Baseline | ||
| < 4.6 | 313 (75.6) | 114 (87.6) | 2.55 | 1.51-4.31 | < 0.001 |
| MCV of RBC (fL) | |||||
| ≤ 100 | 354 (85.5) | 94 (72.3) | Baseline | ||
| > 100 | 60 (14.4) | 36 (27.6) | 2.22 | 1.51-3.26 | < 0.001 |
| MCH9 of RBC (picograms per RBC) | |||||
| ≤ 31 | 242 (58.4) | 51 (39.2) | Baseline | ||
| > 31 | 172 (41.5) | 79 (60.7) | 2.15 | 1.51 - 3.06 | < 0.001 |
| RDW of RBC (%) | |||||
| ≤ 14.5% | 134 (32.3) | 31 (23.8) | Baseline | ||
| > 14.5% | 280 (67.6) | 99 (76.1) | 1.52 | 1.02 - 2.28 | 0.04 |
| Cholesterol (mg/dL) | |||||
| < 200 | 365 (88.1) | 124 (95.3) | Baseline | ||
| ≥ 200 | 49 (11.8) | 6 (4.6) | 0.40 | 0.18 - 0.86 | 0.03 |
| ALT (u/L) | |||||
| < 53 | 274 (66.1) | 63 (48.4) | Baseline | ||
| ≥ 53 | 140 (33.8) | 67 (51.5) | 2.00 | 1.42 - 2.82 | < 0.001 |
| AST (u/L) | |||||
| ≤ 40 | 114 (27.5) | 24 (18.4) | Baseline | ||
| > 40 | 300 (72.4) | 106 (81.5) | 1.79 | 1.15 - 2.79 | 0.01 |
| Alb (g/dL) | |||||
| ≥3.2 | 278 (67.1) | 68 (52.3) | Baseline | ||
| <3.2 | 136 (32.8) | 62 (47.6) | 2.25 | 1.59 - 3.19 | < 0.001 |
| Hepatorenal syndrome | |||||
| No | 413 (99.7) | 127 (97.6) | Baseline | ||
| Yes | 1 (0.2) | 3 (2.3) | 4.21 | 1.33 - 13.27 | 0.014 |
| Hepatopulmonary syndrome | |||||
| No | 413 (99.7) | 127 (97.7) | Baseline | ||
| Yes | 1 (0.2) | 3 (2.3) | 6.74 | 2.13 - 21.33 | 0.001 |
| Myocardial infarction | |||||
| No | 414 (100) | 125 (96.1) | Baseline | ||
| Yes | 0 (0) | 5 (3.8) | 4.41 | 1.80 - 10.80 | 0.001 |
| Weight loss | |||||
| No | 350 (84.5) | 94 (72.3) | Baseline | ||
| Yes | 64 (15.4) | 36 (27.6) | 1.64 | 1.11 - 2.40 | 0.01 |
| Edema | |||||
| No | 316 (76.3) | 77 (59.2) | Baseline | ||
| Yes | 98 (23.6) | 53 (40.7) | 1.57 | 1.11 - 2.40 | 0.01 |
| Upper GI bleeding | |||||
| No | 402 (97.1) | 120 (92.3) | Baseline | ||
| Yes | 12 (2.8) | 10 (7.6) | 1.77 | 0.93 - 3.38 | 0.08 |
| Lethargy | |||||
| No | 263 (63.5) | 59 (45.3) | Baseline | ||
| Yes | 151 (36.4) | 71 (54.6) | 1.83 | 1.30 - 2.59 | 0.001 |
| Hbs Ag | |||||
| Negative | 358 (86.4) | 102 (78.4) | Baseline | ||
| Positive | 56 (13.5) | 28 (21.5) | 1.70 | 1.11 - 2.58 | 0.01 |
| Hbe Ag | |||||
| Negative | 405 (97.8) | 121 (93.0) | Baseline | ||
| Positive | 9 (2.1) | 9 (6.9) | 2.61 | 1.32 - 5.14 | 0.006 |
| Blood lymphocyte (% of WBC) | |||||
| ≥ 24 | 282 (68.1) | 73 (56.1) | Baseline | ||
| < 24 | 132 (31.8) | 57 (43.8) | 1.60 | 1.13 - 2.26 | 0.01 |
| Platelet count (x109/L) | |||||
| ≥ 150,000 | 119 (28.7) | 18 (13.8) | Baseline | ||
| < 150,000 | 295 (71.2) | 112 (86.1) | 2.04 | 1.24 - 3.35 | 0.005 |
| CA 125 (U/mL) | |||||
| ≤ 35 | 130 (31.4) | 16 (12.3) | Baseline | ||
| > 35 | 284 (68.5) | 114 (87.6) | 3.24 | 1.92-5.47 | < 0.001 |
| Total bilirubin (mg/dL) | |||||
| ≤ 1.3 | 78 (18.8) | 20 (15.3) | Baseline | ||
| > 1.3 | 336 (81.1) | 110 (84.6) | 1.63 | 1.01-2.64 | 0.045 |
| BUN (mg/dL) | |||||
| ≤ 25 | 358 (86.4) | 90 (69.2) | Baseline | ||
| > 25 | 56 (13.5) | 40 (30.7) | 2.64 | 1.82-3.84 | < 0.001 |
| Na (meq/L) | |||||
| ≥ 135 | 374 (90.3) | 104 (80.0) | Baseline | ||
| < 135 | 40 (9.6) | 26 (20.0) | 2.55 | 1.66-3.93 | < 0.001 |
| K (meq/L) | |||||
| ≤ 5.5 | 409 (98.7) | 121 (93.0) | Baseline | ||
| > 5.5 | 5 (1.2) | 9 (6.9) | 3.22 | 1.63 - 6.34 | 0.001 |
| Ca (mg/dL) | |||||
| ≥ 8.5 | 362 (87.4) | 97 (74.6) | Baseline | ||
| < 8.5 | 52 (12.5) | 33 (25.3) | 2.13 | 1.44 - 3.17 | < 0.001 |
| MELD score | |||||
| Mean ±SD | 14.9 ± 5.7 | 18.9 ± 6.8 | 1.10 | 1.08 - 1.13 | < 0.001 |
| Median | 15 | 18 | |||
| Fib-4 | |||||
| Mean ± SD | 0.005 ± 0.006 | 0.008 ± 0.011 | 1.621 | 1.3 - 1.9 | < 0.001 |
| Median | 0.004 | 0.006 |
Univariate Analysis of Nutritional Determinants of Mortality in Patients on Waiting List for Liver Transplantation at Shiraz University of Medical Sciences, Iran (Survived = 414, Dead = 130)
| Determinant | Gram | Surviveda | Deada | HR | CI 95% | P-Value |
|---|---|---|---|---|---|---|
| CHO (% of daily nutritional intake) | ||||||
| < 25 | < 139.9 | 75 (18.1) | 37 (28.4) | Baseline | ||
| 25 - 75 | 139.9 - 269.9 | 177 (42.7) | 52 (40) | 0.51 | 0.33 - 0.78 | 0.002 |
| < 75 | > 269.9 | 103 (24.8) | 12 (9.2) | 0.27 | 0.14 - 0.52 | < 0.001 |
| Fat (% of daily nutritional intake) | ||||||
| < 25 | < 20 | 75 (18.1) | 38 (29.2) | Baseline | ||
| 25 - 75 | 20 - 43.1 | 190 (45.8) | 39 (30) | 0.46 | 0.29 - 0.73 | 0.001 |
| < 75 | > 43.1 | 90 (21.7) | 24 (18.4) | 0.56 | 0.33 - 0.93 | 0.02 |
| Protein (% of daily nutritional intake) | ||||||
| < 25 | < 39.2 | 87 (21) | 26 (20) | Baseline | ||
| 25 - 75 | 39.2-72.5 | 168 (40.5) | 60 (46.1) | 1.0 | 0.6 - 1.7 | 0.7 |
| < 75 | > 72.5 | 100 (24.1) | 15 (11.5) | 0.5 | 0.2 - 0.9 | 0.04 |
| Energy, kcal (% of daily need) | ||||||
| < 25 | < 9454 | 76 (18.3) | 37 (28.4) | Baseline | ||
| 25 - 75 | 945 - 1737 | 181 (43.7) | 46 (35.3) | 0.4 | 0.3 - 0.7 | 0.001 |
| < 75 | > 1737 | 98 (23.6) | 18 (13.8) | 0.4 | 0.2 - 0.7 | 0.002 |
Multivariable Analysis of Determinants of Mortality Based on Cox Proportional Hazard and LASSO Cox Regression Models in Patients on Waiting List for Liver Transplantation at Shiraz University of Medical Sciences, Iran
| Variable | Cox Regression | Lasso Regressiona | ||
|---|---|---|---|---|
| HR | SE | HR | SE | |
| Hepatopulmonary syndrome | 4.78 | 0.66 | 1.86 | 0.84 |
| Myocardial infarction | 3.30 | 0.48 | 1.67 | 0.73 |
| Low CHO intake | 2.78 | 0.32 | 1.53 | 0.29 |
| PMN | 2.47 | 0.36 | 1.24 | 0.45 |
| Ca 125 | 2.36 | 0.30 | 1.57 | 0.29 |
| Weight loss | 2.09 | 0.21 | 1.38 | 0.24 |
| ALT | 2.02 | 0.18 | 1.44 | 0.23 |
| Hbs Ag | 1.91 | 0.22 | 1.26 | 0.25 |
| MCV | 1.88 | 0.21 | 1.29 | 0.24 |
| Ascities | 1.56 | 0.20 | 1.15 | 0.16 |
| Gallbladder wall edema (detected by ultrasound) | 1.55 | 0.22 | 1.19 | 0.22 |
| Bun | 1.42 | 0.21 | 1.28 | 0.20 |
| Meld | 1.1 | 0.01 | 1.07 | 0.01 |
Determinants of Survival in the First 3, 6, and 12 Months of Follow-up (from left to right) in Patients on Liver Transplantation Waiting List at Shiraz University of Medical Sciences, Iran (HR: hazard ratio; HBsAg: hepatitis B surface antigen; MCV: mean corpuscular volume; MCH: mean corpuscular hemoglobin; PMN: polymorphonuclear leukocytes; CHO: carbohydrate; MELD: model for end-stage liver disease; RDW: red cell distribution width; SBP: spontaneous bacterial peritonitis; CA 125: cancer antigen 125; MI: myocardial infarction; ALT: alanine aminotransferase; BMI: body mass index).
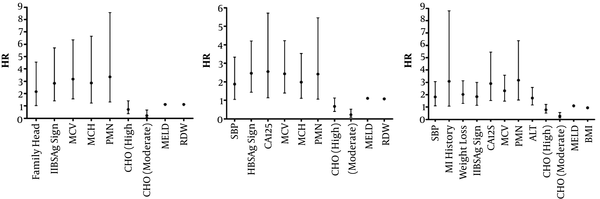
Cumulative Survival of Patients on Liver Transplantation Waiting List at Shiraz University of Medical Sciences, Iran.
5. Discussion
The current study revealed that about one-fourth of the patients on the WL for LT died, while the median time of their survival was five months after enrolling in the WL. It was observed that HPS, history of MI, low-carbohydrate diet, and to a lesser degree, high PMN count, positive serum CA125, weight loss, high level of ALT, positive HBV markers, high MCV of RBCs, ascites, edema of the gallbladder wall, and high level of BUN were the significant determinants of death in LT candidates. These findings could have implications in promoting the care of such patients and revision of allocation strategies in LT.
Chronic liver disease (CLD) and cirrhosis are now silent epidemics, especially in industrial countries, while its prevalence doubled over the past three decades (13). Globally, about 39 million DALYs were related to cirrhosis and CLD in 2016, showing a 37.9% growth in comparison to 1990 (1). Studies found that without appropriate management, nearly all chronic hepatitis types finally progress into ESLD, with possible grave complications, including liver failure, HCC, and death (14). LT is the most effective treatment for ESLD (3, 4). Iran is the 8th country with the highest number of LT in the world, while most cases in this country are accomplished in the Shiraz Organ Transplantation Center(15) . The first orthotropic LT in Shiraz Organ Transplantation Center was performed on 04 May 1993, and a total of 4241 LT were performed in this center till 13 December 2016 (16). However, its demand increased and remained largely more than supply resulting in a long WL. It may increase the mortality of patients on the WL before transplantation (17). Some evidence demonstrated that WL for LT alone did not have any association with Outcome (LT waiting list mortality) (18); therefore, there is a need for more appropriate allocation systems compared to consideration of only waiting time (19). Based on these considerations, MELD scoring was introduced as the main prioritizing instrument for LT in the United States in 2002 and later in other countries, including Iran (20). Many studies thus far evaluated the efficacy of priority systems, such as MELD and Child-Turcotte-Pugh (CTP). Despite the preference in the allocation system, there is no association between baseline MELD and CTP scores with the mortality rate of patients on WL (9), but others proposed the opposite (21, 22). Few studies determined CTP as a better prioritization system (7); however, others showed the MELD score as a better classifier of outcome in such patients (21, 23). A study also showed that the MELD score predicted mortality independent of liver disease etiology and complications (24). Finally, some studies indicated that both systems have equal predictive value for decompensated cirrhosis in daily clinical practice (25). Therefore, the MELD and CTP scoring systems, the two most universally applicable ones, are used in urgency-based allocations. However, there are reports on shortages of both systems and the need to consider other factors to achieve optimal prioritization (19). Different variations of MELD scores, including MESO index, MELD-Na, UKELD, iMELD, refit MELD, refit MELD-Na, upMELD (26), and delta MELD (27), were proposed with different quality results of their prediction of mortality in the liver transplant candidates or post-transplant survival. However, some of these scores lack statistical validity and model evaluation. The introduction of artificial neural network (ANN) in this setting is promising; there are reports on higher accuracy of ANN than the MELD score in the prediction of three-month survival of patients listed for LT (28). The current study findings showed that the MELD score was among the top correlates of mortality in LT candidates. HPS, a MELD exception in another report (29), was the strongest predictor of death. Other factors that had significant associations with mortality in pre-liver transplant patients in the current study were a history of MI and high PMN count. Although studies assessed the impact of pre-transplant heart disease (30) or WBC count (31) on the post-transplant outcome, such an assessment of pre-transplant outcomes was unavailable. In the current study, CA 125 was found as a predictor of mortality in LT candidates. Other studies similarly revealed its association with the severity of cirrhosis, liver decompensation (32), or liver damage, and poor prognosis in patients with the primary Budd-Chiari syndrome (33). It was observed that high MCV of RBCs was a predictor of mortality in LT candidates. Macrocytic anemia was associated with the severity of liver impairment and might be a predictor of short-term mortality in patients with HBV-related decompensated cirrhosis (34).
There were no other studies on the associations of higher ALT levels with the mortality of patients on the WL for LT. Therefore, the current study findings are probably the first reports on such an association.
Despite the importance of nutritional status to the outcome of major operations, including LT, there are few reports on its use to predict pre-transplantation mortality (9). A low-calorie diet in patients with advanced liver disease had a prognostic value, and was associated with higher mortality (35). Previous studies reported that unintentional weight loss due to malnutrition in patients on the WL for LT puts them at the risk of death (9). A study indicated that both underweight (body mass index (BMI) < 18 kg/m2) and obesity were associated with a greater risk of death in LT candidates (36). Another study found that inadequate dietary protein intake was associated with mortality in such patients (9). In the current study, both CPH and LCR models showed that low-carbohydrate diet was associated with the higher mortality rate in the pre-transplantation period. Therefore, it seems necessary to pay continuous attention to the nutritional status of LT candidates to reduce their mortality rate while waiting for LT (37). Totally 11.5 million DALYs and 100,000 death due to HBV infection were reported in 2016 worldwide (1, 2). Viral hepatitis is one of the main indications for LT and a cause of post-transplant poor outcome unless managed appropriately before LT (38). In the current study, both HBsAg and HBeAg had significant associations with the mortality of patients on the WL for LT. However, the effect of these markers on LT candidates’ outcomes was not assessed by other studies. Many studies reported ascites as a cause of early death, even in patients with low MELD scores (39). The current study similarly concluded that ascites had a significant association with outcomes in LT candidates. The study also showed that high BUN and lethargy had significant correlations with the mortality of LT candidates, but no study assessed such variables. It was revealed that edematous thickening of the gallbladder wall was correlated with patient mortality. Another study reported associations between ascites, decreased systemic vascular resistance, and portal hypertension with gallbladder wall thickening in patients with cirrhosis (40). Patient self-health assessment was also among subjective variables that its association with outcome in LT candidates was assessed for the first time and could predict the mortality; however, it should be further validated by future studies.
It was also found that high PMN number, high MCV of RBCs, HBsAg positivity, low-carbohydrate diet, and High MELD score were associated with mortality of patients in the first 3, 6, and 12 months of waiting for LT. Another study showed that iMELD was a more accurate prognostic factor than MELD score during the first 90 days of waiting for LT (26). In the current study, CPH and LCR did not show any significant association between the serum level of sodium and patients mortality.
The main limitation of the current study was the lack of access to medical records of patients who did not participate in the study or lost to follow-up due to wrong phone numbers, not-answering the three phone calls in follow-ups, and cancelation of request for LT. It should be emphasized that the current study was a preliminary report of an ongoing larger-scale cohort study that its findings may reveal more conclusive results.
A multi-central, larger-scale cohort study is highly recommended to find the effect of these factors on WL mortality rate and post-transplantation survival and complications. To achieve more accurate results, comparative studies with both linear and non-linear models to predict such patients’ outcomes are required.
A significant proportion of patients died while waiting for LT. To avoid or reduce this mortality, the allocation strategies should also include other factors, besides MELD, such as HPS, history of MI, low CHO intake, weight loss, ascites, and paraclinical markers, such as PMN, CA 125, ALT, HBsAg, MCV, BUN, and gallbladder wall thickness.
References
-
1.
DALYs GBD, Collaborators H. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1260-344. [PubMed ID: 28919118]. https://doi.org/10.1016/s0140-6736(17)32130-x.
-
2.
Evaluation IfHMa. Deaths and DALYs for Cirrhosis and other chronic liver diseases for 2016 with trends since 1990 2017. 2019, [cited 5 November 2019]. Available from: http://ghdx.healthdata.org/gbd-results-tool?params=gbd-api-2016-permalink/b1b912cfcf5a6f0d23d3eadfc466272b.
-
3.
Muller X, Marcon F, Sapisochin G, Marquez M, Dondero F, Rayar M, et al. Defining benchmarks in liver transplantation: A multicenter outcome analysis determining best achievable results. Ann Surg. 2018;267(3):419-25. [PubMed ID: 28885508]. https://doi.org/10.1097/SLA.0000000000002477.
-
4.
Zamora-Valdes D, Leal-Leyte P, Kim PT, Testa G. Fighting mortality in the waiting list: Liver transplantation in north America, Europe, and Asia. Ann Hepatol. 2017;16(4):480-6. [PubMed ID: 28612751]. https://doi.org/10.5604/01.3001.0010.0271.
-
5.
Fisher RA. Living donor liver transplantation: eliminating the wait for death in end-stage liver disease? Nat Rev Gastroenterol Hepatol. 2017;14(6):373-82. [PubMed ID: 28196987]. https://doi.org/10.1038/nrgastro.2017.2.
-
6.
Saberifiroozi M, Serati AR, Malekhosseini SA, Salahi H, Bahador A, Lankarani KB, et al. Analysis of patients listed for liver transplantation in Shiraz, Iran. Indian J Gastroenterol. 2006;25(1):11-3. [PubMed ID: 16567887].
-
7.
Gotthardt D, Weiss KH, Baumgartner M, Zahn A, Stremmel W, Schmidt J, et al. Limitations of the MELD score in predicting mortality or need for removal from waiting list in patients awaiting liver transplantation. BMC Gastroenterol. 2009;9:72. [PubMed ID: 19778459]. [PubMed Central ID: PMC2760571]. https://doi.org/10.1186/1471-230X-9-72.
-
8.
Merion RM, Wolfe RA, Dykstra DM, Leichtman AB, Gillespie B, Held PJ. Longitudinal assessment of mortality risk among candidates for liver transplantation. Liver Transpl. 2003;9(1):12-8. [PubMed ID: 12514767]. https://doi.org/10.1053/jlts.2003.50009.
-
9.
Ferreira LG, Anastacio LR, Lima AS, Touslon Davisson Correia MI. Predictors of mortality in patients on the waiting list for liver transplantation. Nutr Hosp. 2013;28(3):914-9. [PubMed ID: 23848119]. https://doi.org/10.3305/nh.2013.28.3.6333.
-
10.
Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46(1):32-6. [PubMed ID: 17567829]. https://doi.org/10.1002/hep.21669.
-
11.
Raeisi Shahraki H, Pourahmad S, Ayatollahi SM. Identifying the prognosis factors in death after liver transplantation via adaptive LASSO in Iran. J Environ Public Health. 2016;2016:7620157. [PubMed ID: 27648080]. [PubMed Central ID: PMC5014976]. https://doi.org/10.1155/2016/7620157.
-
12.
Shahraki HR, Salehi A, Zare N. Survival prognostic factors of male breast cancer in southern Iran: a LASSO-Cox regression approach. Asian Pac J Cancer Prev. 2015;16(15):6773-7. [PubMed ID: 26434910]. https://doi.org/10.7314/apjcp.2015.16.15.6773.
-
13.
Doycheva I, Watt KD, Rifai G, Abou Mrad R, Lopez R, Zein NN, et al. Increasing burden of chronic liver disease among adolescents and young adults in the USA: A silent epidemic. Dig Dis Sci. 2017;62(5):1373-80. [PubMed ID: 28194666]. https://doi.org/10.1007/s10620-017-4492-3.
-
14.
Wang FS, Fan JG, Zhang Z, Gao B, Wang HY. The global burden of liver disease: the major impact of China. Hepatology. 2014;60(6):2099-108. [PubMed ID: 25164003]. [PubMed Central ID: PMC4867229]. https://doi.org/10.1002/hep.27406.
-
15.
Matesanz R. Donation and transplantation activity. Transplant newsletter 2016. 2019, [cited 3 November 2019]. Available from: https://www.swissinfo.ch/blob/43613866/825b24970155b5877fba0263f928dba7/newsletter-transplant--international-figures-on-donation-and-transplantation-2016-data.pdf.
-
16.
Malek Hosseini SA, Nikeghbalian S, Salahi H, Kazemi K, Shemsaeifar A, Bahador A, et al. Evolution of liver transplantation program in Shiraz, Iran. Hepat Mon. 2017;17(11). https://doi.org/10.5812/hepatmon.60745.
-
17.
Gheorghe L, Popescu I, Iacob R, Iacob S, Gheorghe C. Predictors of death on the waiting list for liver transplantation characterized by a long waiting time. Transpl Int. 2005;18(5):572-6. [PubMed ID: 15819806]. https://doi.org/10.1111/j.1432-2277.2005.00090.x.
-
18.
Freeman RJ, Edwards EB. Liver transplant waiting time does not correlate with waiting list mortality: implications for liver allocation policy. Liver Transpl. 2000;6(5):543-52. [PubMed ID: 10980052]. https://doi.org/10.1053/jlts.2000.9744.
-
19.
Schilsky ML, Moini M. Advances in liver transplantation allocation systems. World J Gastroenterol. 2016;22(10):2922-30. [PubMed ID: 26973389]. [PubMed Central ID: PMC4779916]. https://doi.org/10.3748/wjg.v22.i10.2922.
-
20.
Llado L, Figueras J, Memba R, Xiol X, Baliellas C, Vazquez S, et al. Is MELD really the definitive score for liver allocation? Liver Transpl. 2002;8(9):795-8. [PubMed ID: 12200780]. https://doi.org/10.1053/jlts.2002.34637.
-
21.
Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33(2):464-70. [PubMed ID: 11172350]. https://doi.org/10.1053/jhep.2001.22172.
-
22.
Fink MA, Berry SR, Gow PJ, Angus PW, Wang BZ, Muralidharan V, et al. Risk factors for liver transplantation waiting list mortality. J Gastroenterol Hepatol. 2007;22(1):119-24. [PubMed ID: 17201891]. https://doi.org/10.1111/j.1440-1746.2006.04422.x.
-
23.
Brown RJ, Kumar KS, Russo MW, Kinkhabwala M, Rudow DL, Harren P, et al. Model for end-stage liver disease and Child-Turcotte-Pugh score as predictors of pretransplantation disease severity, posttransplantation outcome, and resource utilization in United Network for Organ Sharing status 2A patients. Liver Transpl. 2002;8(3):278-84. [PubMed ID: 11910574]. https://doi.org/10.1053/jlts.2002.31340.
-
24.
Wiesner RH, McDiarmid SV, Kamath PS, Edwards EB, Malinchoc M, Kremers WK, et al. MELD and PELD: application of survival models to liver allocation. Liver Transpl. 2001;7(7):567-80. [PubMed ID: 11460223]. https://doi.org/10.1053/jlts.2001.25879.
-
25.
Papatheodoridis GV, Cholongitas E, Dimitriadou E, Touloumi G, Sevastianos V, Archimandritis AJ. MELD vs Child-Pugh and creatinine-modified Child-Pugh score for predicting survival in patients with decompensated cirrhosis. World J Gastroenterol. 2005;11(20):3099-104. [PubMed ID: 15918197]. [PubMed Central ID: PMC4305847]. https://doi.org/10.3748/wjg.v11.i20.3099.
-
26.
Saldana RS, Schrem H, Barthold M, Kaltenborn A. Prognostic abilities and quality assessment of models for the prediction of 90-day mortality in liver transplant waiting list patients. PLoS One. 2017;12(1). e0170499. [PubMed ID: 28129338]. [PubMed Central ID: PMC5271345]. https://doi.org/10.1371/journal.pone.0170499.
-
27.
Zakareya T, Abbasy M, Abdel-Razek W, Elsiesy H, Abal Khail F, Al Sebayel M, et al. Utility of post-liver transplantation MELD and delta MELD in predicting early and late mortality. Eur J Gastroenterol Hepatol. 2017;29(12):1424-7. [PubMed ID: 28957872]. https://doi.org/10.1097/MEG.0000000000000957.
-
28.
Cucchetti A, Vivarelli M, Heaton ND, Phillips S, Piscaglia F, Bolondi L, et al. Artificial neural network is superior to MELD in predicting mortality of patients with end-stage liver disease. Gut. 2007;56(2):253-8. [PubMed ID: 16809421]. [PubMed Central ID: PMC1856758]. https://doi.org/10.1136/gut.2005.084434.
-
29.
Choden T, Satoskar R. Meld-na: Does this leave anyone behind? Curr Hepatol Rep. 2017;16(3):220-7. https://doi.org/10.1007/s11901-017-0356-8.
-
30.
VanWagner LB, Serper M, Kang R, Levitsky J, Hohmann S, Abecassis M, et al. Factors associated with major adverse cardiovascular events after liver transplantation among a national sample. Am J Transplant. 2016;16(9):2684-94. [PubMed ID: 26946333]. [PubMed Central ID: PMC5215909]. https://doi.org/10.1111/ajt.13779.
-
31.
Helfritz F, Lehner F, Manns MP. Perioperative white blood cell count as a marker for patient and graft survival after orthotopic liver transplantation. J Hepatol Gastroint Dis. 2015;1(1). https://doi.org/10.4172/2475-3181.1000106.
-
32.
Edula RG, Muthukuru S, Moroianu S, Wang Y, Lingiah V, Fung P, et al. Ca-125 significance in cirrhosis and correlation with disease severity and portal hypertension: A retrospective study. J Clin Transl Hepatol. 2018;6(3):241-6. [PubMed ID: 30271734]. [PubMed Central ID: PMC6160305]. https://doi.org/10.14218/JCTH.2017.00070.
-
33.
Cheng DL, Xu H, Lv WF, Hua R, Du H, Zhang QQ. The significance of serum CA-125 elevation in chinese patients with primary budd-chiari syndrome: A multicenter study. Gastroenterol Res Pract. 2015;2015:121060. [PubMed ID: 26451141]. [PubMed Central ID: PMC4587407]. https://doi.org/10.1155/2015/121060.
-
34.
Yang J, Yan B, Yang L, Li H, Fan Y, Zhu F, et al. Macrocytic anemia is associated with the severity of liver impairment in patients with hepatitis B virus-related decompensated cirrhosis: a retrospective cross-sectional study. BMC Gastroenterol. 2018;18(1):161. [PubMed ID: 30384828]. [PubMed Central ID: PMC6211489]. https://doi.org/10.1186/s12876-018-0893-9.
-
35.
Cabre E, Gonzalez-Huix F, Abad-Lacruz A, Esteve M, Acero D, Fernandez-Banares F, et al. Effect of total enteral nutrition on the short-term outcome of severely malnourished cirrhotics. A randomized controlled trial. Gastroenterology. 1990;98(3):715-20. [PubMed ID: 2105256]. https://doi.org/10.1016/0016-5085(90)90293-a.
-
36.
Dick AA, Spitzer AL, Seifert CF, Deckert A, Carithers RJ, Reyes JD, et al. Liver transplantation at the extremes of the body mass index. Liver Transpl. 2009;15(8):968-77. [PubMed ID: 19642131]. https://doi.org/10.1002/lt.21785.
-
37.
Ghaemi A, Hosseini N, Osati S, Naghizadeh MM, Dehghan A, Ehrampoush E, et al. Waist circumference is a mediator of dietary pattern in Non-alcoholic fatty liver disease. Sci Rep. 2018;8(1):4788. [PubMed ID: 29555959]. [PubMed Central ID: PMC5859081]. https://doi.org/10.1038/s41598-018-23192-x.
-
38.
Curry MP. Hepatitis B and hepatitis C viruses in liver transplantation. Transplantation. 2004;78(7):955-63. [PubMed ID: 15480158]. https://doi.org/10.1097/01.tp.0000140927.63952.58.
-
39.
Sanyal AJ, Genning C, Reddy KR, Wong F, Kowdley KV, Benner K, et al. The north american study for the treatment of refractory ascites. Gastroenterology. 2003;124(3):634-41. [PubMed ID: 12612902]. https://doi.org/10.1053/gast.2003.50088.
-
40.
Wang TF, Hwang SJ, Lee EY, Tsai YT, Lin HC, Li CP, et al. Gall-bladder wall thickening in patients with liver cirrhosis. J Gastroenterol Hepatol. 1997;12(6):445-9. [PubMed ID: 9195402]. https://doi.org/10.1111/j.1440-1746.1997.tb00464.x.