Abstract
Background:
Adnexal mass is one of the most common gynecologic diseases among women of all ages.Methods:
In this cross-sectional study, we enrolled 126 patients with large adnexal masses (≥ 10 cm) managed by laparoscopic surgery during 2013 - 2020. The rates of intraoperative complications, conversion to open surgery, and incidence of cancer were assessed.Results:
Mean mass size was 15.08 ± 5.03 in all participants without significant difference based on the tumor type (P = 0.624). Mean age and operation time were higher in the malignant type compared to the benign type (P < 0.001). Type of surgery and frequency of intraoperative complications were also different among patients with different tumor types (P < 0.001 and P = 0.816, respectively).Conclusions:
Our study showed that large adnexal tumors can be operated by laparoscopic approach, while the most important factor for increased surgical complications and duration was malignancy.Keywords
1. Background
Adnexal mass is one of the most common gynecologic diseases among women of all ages, especially during reproductive ages, with an estimated prevalence of 5 - 10% in different populations (1). The gold standard management for benign ovarian masses is laparoscopy with surgical outcomes similar to laparotomy; in addition, it has several benefits to laparotomy, including faster recovery, less and shorter postoperative pain, reduced inpatient admission, and superior cosmetic outcomes (2). However, laparoscopic management of adnexal masses has some limitations, such as difficulty in inserting trocars and limited surgical field that inhibits the complete exploration of the abdominal cavity, as well as the risk of ovarian rupture and spread of cyst fluid into the abdominal cavity (3). While some studies suggest that the iatrogenic or accidental rupture and spillage of the malignant adnexal mass contents upgrades the tumor stage and decreases the overall survival (4), some others suggest that it has no adverse prognostic significance (5). Although the laparoscopic operation would be much easier and faster after puncturing the benign cysts, the risk of spillage of malignant cells hinders the surgeons from puncturing the cyst for ease of operation, which results in preference for open surgery (6). Meanwhile, it is not clear whether the risk of rupture differs based on the tumor type, which has to be further investigated.
Another important factor for the choice of laparoscopic or laparotomic management of adnexal masses is the size of the mass. Moreover, some studies suggest the use of laparotomy in large adnexal masses due to the technical difficulty of performing laparoscopy, limited surgical field, and the higher probability of malignant potential in adnexal masses (7, 8). However, the cut-off level for the “giant”, “huge”, and “large” tumors are not clearly defined, and no contraindications have been defined for the laparoscopic approach based on maximum tumor size (5, 9). Despite many restrictions for laparoscopic approach in large adnexal masses, recent literature has suggested that many of these adnexal masses can still be managed using laparoscopy (10). Some have also suggested that tumor size is not a predictor of perioperative complications (11). Due to the complications of laparotomy, it is worth to examine the applicability of laparoscopy in large adnexal masses. Considering the controversies regarding the treatment of large ovarian tumors with laparoscopic surgery, the present study aimed to report the surgical outcome of patients with large (≥ 10 cm) adnexal masses undergoing laparoscopic surgery and factors associated with the surgical outcome.
2. Methods
This cross-sectional study was conducted at Shohadaye-Tajrish Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran. All women with an adnexal mass of ≥ 10 cm based on preoperative ultrasonography and magnetic resonance imaging (MRI), who underwent laparoscopic surgery from July 2013 to January 2020, were enrolled in the study. The study was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences (SBMU.RETECH.REC1397.1032). Informed consent was obtained from all participants for participation in the study and conversion to laparotomy in case of unexpected complications or incidental malignancy finding. A total of 126 participants who met the inclusion criteria were enrolled in the study by census method.
All patients underwent preoperative physical examination and imaging. Abdominal and vaginal ultrasound was performed by an expert; the mass size was characterized based on the maximum diameter reported in the imaging studies. One venous blood sample was obtained from all patients and sent to the laboratory for measurement of tumor markers, including lactate dehydrogenase (LDH), cancer antigen (CA) 19-9, CA125, carcinoma embryonic antigen (CEA), human epididymis protein 4 (HE4), inhibin, and beta-HCG were measured in all patients prior to surgery. Those patients who had ascites or metastasis, obesity, pregnancy, a previous history of abdominal surgery, and a probable malignancy without metastasis (mass features in imaging or elevated tumor markers) were not included. Patients with any amounts of tumor marker were included in the study. Patients with metastasis and ascites were excluded.
All the surgeries were performed by a single surgeon (B. Nouri) under general anesthesia with a single surgical protocol. For this purpose, patients were laid in the dorsal lithotomy position, and after induction of general anesthesia, a 10-mm trocar was inserted either directly or using an altered open technique at the umbilicus, subxiphoid, or palmar site, according to the tumor size. Other trocars were placed under direct visualization. First, the whole abdomen and pelvis were inspected. Subsequently, peritoneal fluid was sent for cytological assessment. Peritoneal washing was performed in case of no peritoneal fluid. The ovarian masses were removed with caution to remain intact and placed within endo-bag using a 15-cm opening diameter (Endo Catch II TM, Covidien Tyco, Norwalk, CT, USA). If we decided to initiate the drainage of very large masses, we performed cystectomy or oophorectomy with or without salpingectomy based on the patients’ age, medical history, and intraoperative findings. The cyst walls were punctured by either of the following methods. In the first method, we drained the cyst fluid using a 5-mm trocar with sleeve inserted into cyst wall dome in laparoscopic visualization; then, we removed the trocar and placed a suction-irrigation device into the cyst wall through the sleeve. In the second approach, the cyst wall was incised between two graspers, and the mass was inserted with the suction-irrigation device. The mass was drained as much as possible, and the puncture site was immediately closed by a grasper to avoid leakage. Homeostasis was achieved and the access site for specimen retrieval and the umbilical port site were closed in different anatomical layers.
The management plans were made based on the frozen section findings. The surgical staging was performed by laparoscopy, and conversion to laparotomy was conducted only for cases with technical difficulties. Intraoperative and postoperative complications, conversion to laparotomy, type of procedure, operation time, and pathologic reports were documented. Operative time was recorded as the time from skin incision to skin closure. Blood loss was measured based on the fluid gathered in the suction bottle. Spillage was characterized as any deliberate or accidental mass wall rupture. If the tumor was drained intentionally into the endo-bag without a peritoneal spill, the tumor was not regarded as a ruptured tumor.
2.1. Statistical Analysis
Statistical analysis was performed using Excel program, 2007 version. The Kolmogorov-Smirnov test was used to examine normal distribution of the data. One-way analysis of variance (ANOVA) was applied to determine the differences among the groups; in case of significance, pairwise comparisons were performed using Tukey’s test. Fisher’s exact test was applied to analyze ratio, and chi-square test was used for categorical variables. P-values less than 0.05 were considered significant.
3. Results
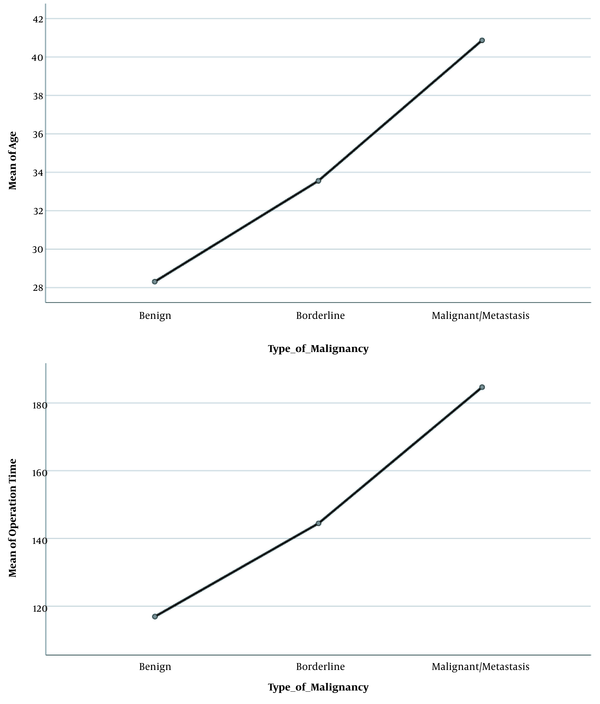
A total of 126 women completed the study. Patients’ demographic characteristics are shown in Table 1. The mean age of the patients was 30.21 ± 11.64 years, 80.2% had benign tumor type (N = 101), 7.1% borderline (N = 10), and 11.9% malignant/metastasis (N = 15). There was a significant difference among the three groups in terms of mean age and operation time; age and duration of surgery were higher in the malignant type compared to the benign type (P < 0.001; Table 1). However, the mean body mass index (BMI), number of deliveries, bleeding volume, and fluid volume drained from the mass were not different based on the tumor type (P > 0.05; Table 1). Mean mass size was 15.08 ± 5.03 in all participants without significant difference based on the tumor type (P = 0.624; Table 1). Figure 1 shows the comparison of mean age and duration of surgery in the patients with three tumor types.
The Demographic Characteristics of the Study Participants Categorized Based on Tumor Type a
| Variables | Total (N = 126) | Benign (N = 101) | Borderline (N = 9) | Malignant or Metastasis (N = 15) | P-Value Among the Three Groups b | P-Value Benign-Borderline c | P-Value Benign-Malignant c | P-Value Malignant-Borderline c |
|---|---|---|---|---|---|---|---|---|
| Age (y) | 30.21 ± 11.64 | 28.31 ± 10.27 | 33.56 ± 16.78 | 40.87 ± 11.76 | < 0.001 | 0.359 | < 0.001 | 0.260 |
| BMI (kg/m2) | 25.95 ± 6.12 | 26.03 ± 6.10 | 25.55 ± 5.15 | 26.20 ± 6.98 | 0.968 | 0.973 | 0.994 | 0.967 |
| Parity (No.) | 2.3 ± 1.2 | 0.78 ± 1.11 | 0.56 ± 1.13 | 1.13 ± 1.12 | 0.414 | 0.830 | 0.946 | 0.442 |
| Mass size (cm) | 15.29 ± 5.09 | 15.08 ± 5.03 | 16.33 ± 6.34 | 16.13 ± 5.08 | 0.624 | 0.763 | 0.739 | 0.995 |
| Duration of surgery (min) | 128.88 ± 63.37 | 116.92 ± 52.08 | 144.44 ± 92.88 | 184.67 ± 56.67 | < 0.001 | 0.340 | < 0.001 | 0.210 |
| Bleeding volume (cc) | 124.61 ± 287.32 | 105.02 ± 247.07 | 84.56 ± 68.38 | 275.53 ± 519.41 | 0.092 | 0.977 | 0.082 | 0.254 |
| Fluid volume drained from the mass (cc) | 1687.27 ± 928.37 | 1674.51 ± 948.86 | 2050.00 ± 353.55 | 1650.00 ± 1060.66 | 0.858 | 0.846 | 0.999 | 0.906 |
Comparison of mean age and duration of surgery in the patients with three tumor types
The first trocar was inserted in the umbilicus in 27.8% (N = 35), upper umbilicus in 21.4% (N = 27), subxiphoid in 48% (N = 61), and palmar in 2.8% (N = 3) of patients. Seventy-five patients (59.5%) were nulliparous, 17 (13.5%) had one child, and 34 (27%) had two or more children. The results of serum parameters are shown in Table 2.
The Frequency of Tumor Markers in the Study Participants
| Tumor Markers | Number | Percentage |
|---|---|---|
| Normal | 75 | 59.5 |
| High CA125 | 26 | 20.6 |
| High CA125 + high HE4 | 1 | 0.8 |
| High CA125 + high CA19-9 | 4 | 3.2 |
| High HE4 | 13 | 10.3 |
| High HE4 + high inhibin + high CA19-9 | 1 | 0.8 |
| High inhibin | 2 | 1.6 |
| High LDH | 1 | 0.8 |
| High LDH + high CA19-9 | 1 | 0.8 |
| High CA19-9 | 1 | 0.8 |
| Positive BHCG | 1 | 0.8 |
Twelve (9.5%) patients were menopause, and there was a significant difference in the frequency of menopause among patients with different tumor types (P = 0.002; Table 3). The type of surgery and frequency of intraoperative complications were also different among patients with different tumor types (P < 0.001 and 0.003, respectively; Table 3), but the frequency of tumor type and tumor site, site of the first trocar, history of abdominal surgery, and blood infusion were not different in patients with different tumor types (P > 0.05; Table 3).
The Frequency of Surgical and Tumor Characteristics in the Study Participants Categorized Based on Tumor Type a, b
| Variables | Type of Malignancy | P-Value | |||
|---|---|---|---|---|---|
| Total (N = 126) | Benign (N = 101) | Borderline (N = 9) | Malignant/Metastasis (N = 15) | ||
| Tumor type | 0.221 | ||||
| Cystic | 72 (57.1) | 61 (60.4) | 4 (44.4) | 6 (40.0) | |
| Solid-cystic | 52 (41.3) | 39 (38.6) | 5 (55.6) | 8 (53.3) | |
| Solid | 3 (2.4) | 1 (1.0) | 0 (0.0) | 1 (6.7) | |
| Tumor side | 0.258 | ||||
| Unilateral | 97 (77.0) | 78 (82.1) | 7 (77.8) | 11 (78.6) | |
| Bilateral | 21 (16.7) | 17 (17.9) | 2 (22.2) | 2 (14.3) | |
| Type of surgery | < 0.001 | ||||
| Cystectomy | 76 (63.2) | 73 (645.2) | 2 (22.2) | 0 (0.0) | |
| Cystectomy + staging | 1 (0.8) | 0 (0.0) | 0 (0.0) | 1 (6.7) | |
| Salpingo-oophorectomy | 31 (24.6) | 25 (23.8) | 0 (0.0) | 6 (33.4) | |
| TLH + BSO + omentectomy + appendectomy | 1 (0.8) | 1 (0.8) | 0 (0.0) | 0 (0.0) | |
| lh + bilateral oophorectomy + staging | 8 (6.4) | 0 (0.0) | 4 (44.4) | 4 (26.7) | |
| Bilateral oophorectomy + staging | 5 (4.0) | 0 (0.0) | 2 (22.2) | 3 (20.0) | |
| TAH + BSO + cytoreductive surgery | 1 (0.8) | 0 (0.0) | 0 (0.0) | 1 (0.8) | |
| Mass resection + adhesion release | 5 (4.0) | 3 (3.0) | 0 (0.0) | 2 (13.3) | |
| First trocar site | 0.527 | ||||
| Umbilicus | 35 (27.8) | 28 (27.7) | 1 (11.1) | 6 (40.0) | |
| Upper umbilicus | 27 (21.4) | 19 (18.8) | 3 (33.3) | 5 (33.3) | |
| Sub xiphoid | 60 (48.0) | 50 (49.5) | 5 (55.6) | 5 (33.3) | |
| Palmar | 3 (2.8) | 2 (2.0) | 1 (11.1) | 0 (0.0) | |
| Intraoperative complications | 0.816 | ||||
| None | 107 (84.9) | 90 (89.1) | 8 (88.9) | 8 (53.3) | |
| Accidental cyst rupture | 6 (4.8) | 4 (4.0) | 0 (0.0) | 2 (14.3) | |
| Pelvic abscess | 2 (1.6) | 0 (0.0) | 0 (0.0) | 2 (14.3) | |
| Intestinal perforation | 1 (0.8) | 0 (0.0) | 1 (11.1) | 0 (0.0) | |
| Intestinal serous trauma | 1 (0.8) | 1 (1.0) | 0 (0.0) | 0 (0.0) | |
| Blood transfusion | 5 (4.0) | 5 (5.0) | 0 (0.0) | 0 (0.0) | |
| Conversion to laparotomy | 2 (1.6) | 0 (0.0) | 0 (0.0) | 2 (14.2) | |
| Rupture of bladder | 1 (0.8) | 1 (1.0) | 0 (0.0) | 0 (0.0) | |
| Umbilical infection | 1 (0.8) | 0 (0.0) | 0 (0.0) | 1 (7.1) | |
| History of surgery | 0.687 | ||||
| No | 75 (59.5) | 63 (62.4) | 5 (55.6) | 7 (50.0) | |
| Yes | 50 (40.5) | 38 (37.6) | 4 (44.4) | 7 (50.0) | |
| Blood infusion | 0.109 | ||||
| No | 117 (92.9) | 96 (95.0) | 9 (100.0) | 11 (78.6) | |
| Yes | 7 (5.6) | 5 (5.0) | 0 (0.0) | 2 (14.3) | |
| Menopause | 0.002 | ||||
| No | 111 (88.1) | 93 (94.9) | 7 (77.8) | 10 (66.7) | |
| Yes | 12 (9.5) | 5 (5.1) | 2 (22.2) | 5 (33.3) | |
4. Discussion
In this study, we reported the surgical outcome of 126 patients with adnexal tumors ≥ 10 cm (mean of 15.08 ± 5.03 cm; maximum of 30 cm), among whom only one required conversion to open surgery. These results suggest the feasibility of laparoscopic management of adnexal masses ≥ 10 cm. In another study on 77 women with ovarian cysts ≥ 10 cm, conversion to open surgery was required in four patients (12), which is higher than that of ours. This difference could be due to the difference in the experience of the laparoscopic surgeon and the difference in the rate of intraoperative complications between the studies. In the study by Vlahos et al., among 19 patients who had adnexal mass with a mean diameter of 8.3 cm, there were no cases of conversion to open surgery (13). The difference between the results of this study and that of ours could be due to the small sample size of their study; however, this study confirms the results of the present study, considering the low risk of conversion to open surgery in large adnexal masses.
One of the important factors against the suggestion of laparoscopic surgery for large adnexal masses is the risk of rupture and its negative effect on patients’ outcomes (7, 8). However, in our study, incidental rupture was only observed in eight patients, two of whom were malignant. These results show that the laparoscopic method has an acceptable rate of cyst rupture. In another study by Shiota et al., comparison of 1,483 cases of benign ovarian cysts according to the cyst size showed no difference in the incidence of cyst rupture among patients with cyst sizes < 5 cm, 5 - 10 cm, and > 10 cm (14), which confirm the results of the present study. In another study by Detorakis et al., studying the surgical outcome of 102 women with adnexal cysts with mean size of 5.7 cm (2.3 - 10.5 cm) showed cyst rupture in 31.8% of the patients and 7.2% in masses > 8 cm (15). These authors concluded that laparotomy is the preferred method for large adnexal masses, but generally speaking, iatrogenic or accidental rupture and spillage of the adnexal mass contents are considered as an inevitable incidence during surgery and may occur both in laparoscopic and laparotomic approaches (4). Furthermore, the prognostic value of significant spillage in malignant cases is still controversial, and some suggest that laparoscopic treatment of ovarian cancer does not have a higher risk of spillage (16). Therefore, we believe that the risk of rupture should not ban surgeons from the choice of laparoscopy, considering the other advantages of this method. With the availability of frozen sections at many tertiary centers and adherence to proper surgical techniques, the chance of spreading malignancy has been reduced considerably.
Another important surgical complication is the bleeding volume during surgery and requirement of blood transfusion, and the results showed that the mean bleeding volume of the studied patients was 124.61 ± 287.32 cc, and only five patients required blood transfusion. In another study by Demir and Marchand, the results showed that 97.8% of women with adnexal masses of 8 - 13 cm treated with laparoscopy had blood loss of < 200 cc (9). These results confirm the findings of the present study on the low bleeding in laparoscopic treatment of large adnexal masses, which is considered as one of the important advantages of laparoscopy vs. laparotomy (17, 18). The mean operation time was 128.88 ± 63.37 minutes in our study. In the study by Vlahos et al. on 53 women with adnexal masses of all sizes undergoing laparoscopy, the mean operative time was 45 minutes (13), which is much less than that of the present study. In the study by Demir and Marchand, 97.92% of surgeries lasted < 136 minutes (9), which is similar to the results of the present study. While they only evaluated patients with benign type, we included patients with any pathologic type. In the study by Machida et al., comparison of the median operation time was significantly higher in cases with adnexal masses > 10 cm vs. < 5 cm (73 vs. 59 minutes) (11). These results suggest that large adnexal masses can prolong the surgical duration, which is justifiable by the technical difficulty of laparoscopy in large masses, which is considered one of the disadvantages of this approach for these cases. However, the results of our study showed that the risk of surgical complications is not serious when patients are selected after complete physical examination, precise imaging studies, and measurement of tumor markers. Furthermore, all surgeries were carried out by a single surgeon; in the meantime, a multidisciplinary team of experts consisting of pathologists, oncologists, colorectal surgeons, and urologists were involved and ready to be called on when necessary. As suggested, the risk of surgical complications is not predicted by the tumor size (11). Therefore, it does not seem logical to impose patients to the critical risk of invasive open surgeries, especially in cases with benign pathologies. The maximum tumor size for safe laparoscopic approach is yet to be determined, as 10 cm threshold seems questionable.
The incidence rate of incidental findings of ovarian cancer during laparoscopy has been reported to be between 0.65 and 0.9% in premenopausal women and 3% in postmenopausal women (19). In our study, there were 15 patients with malignancy or metastasis, 5 (33.3%) of whom were postmenopausal. Other studies have reported other incidence rates for malignant ovarian mass (4, 9, 12), which can vary based on the frequency of malignancy in the study place and based on the inclusion criteria of the study. The results showed that patients with malignancy or metastasis were significantly older and had a longer duration of surgery and intraoperative complications. These results are consistent with the results of the study by Gad et al., which reported higher rate of complications and longer operative time in patients with borderline/malignant adnexal mass, compared to the benign group undergoing laparoscopic treatment (20). Furthermore, they reported a higher rate of conversion to open surgery, blood loss, and duration of hospital stay (20), which was not observed in our study. Other studies have also confirmed the superiority of laparoscopy vs. laparotomy for treatment of ovarian cancers (21, 22), as well as comparable accuracy of staging of laparoscopy vs. laparotomy and comparable survival rates (23, 24), while the results of the present study suggested higher complication rates in large tumors. Due to the small sample size of this subgroup in our study, further studies should be performed to investigate the applicability of laparoscopy in large adnexal malignant tumors.
Our study had some limitations. The first limitation was the cross-sectional nature of this study, which limited suggestion of causal relationship between the study variables. Furthermore, we did not follow patients to study the long-term results and did not evaluate the survival or recurrence rate in the studied population. The small sample of the study, especially in subgroups, was another limitation of the present study.
4.1. Conclusions
Our study showed that the size of tumor alone might not be a limiting factor for using laparoscopic approach in the treatment of adnexal masses, as it resulted in acceptable rates of intraoperative complications. These results suggest the safety and efficacy of laparoscopic approach for large adnexal masses, when performed by an expert laparoscopic surgeon on patients. As higher rates of surgical complications and longer operation time were only observed in patients with malignancy or metastasis, due to the small sample size of this subgroup in our study, more studies are required to investigate the feasibility and safety of laparoscopic treatment of this subgroup of large adnexal masses.
References
-
1.
Pavlik EJ, Ueland FR, Miller RW, Ubellacker JM, DeSimone CP, Elder J, et al. Frequency and disposition of ovarian abnormalities followed with serial transvaginal ultrasonography. Obstet Gynecol. 2013;122(2 Pt 1):210-7. [PubMed ID: 23969786]. https://doi.org/10.1097/AOG.0b013e318298def5.
-
2.
Liu JH, Zanotti KM. Management of the adnexal mass. Obstet Gynecol. 2011;117(6):1413-28. [PubMed ID: 21606754]. https://doi.org/10.1097/AOG.0b013e31821c62b6.
-
3.
Hoorsan H, Alavi Majd H, Chaichian S, Mehdizadehkashi A, Hoorsan R, Akhlaqghdoust M, et al. Maternal anthropometric characteristics and adverse pregnancy outcomes in iranian women: A confirmation analysis. Arch Iran Med. 2018;21(2):61-6. [PubMed ID: 29664656].
-
4.
Matsushita H, Watanabe K, Yokoi T, Wakatsuki A. Unexpected ovarian malignancy following laparoscopic excision of adnexal masses. Hum Reprod. 2014;29(9):1912-7. [PubMed ID: 24964925]. https://doi.org/10.1093/humrep/deu162.
-
5.
Alobaid A, Memon A, Alobaid S, Aldakhil L. Laparoscopic management of huge ovarian cysts. Obstet Gynecol Int. 2013;2013:380854. [PubMed ID: 23766763]. [PubMed Central ID: PMC3665257]. https://doi.org/10.1155/2013/380854.
-
6.
Kim HS, Ahn JH, Chung HH, Kim JW, Park NH, Song YS, et al. Impact of intraoperative rupture of the ovarian capsule on prognosis in patients with early-stage epithelial ovarian cancer: A meta-analysis. Eur J Surg Oncol. 2013;39(3):279-89. [PubMed ID: 23273873]. https://doi.org/10.1016/j.ejso.2012.12.003.
-
7.
Nezhat C, Cho J, King LP, Hajhosseini B, Nezhat F. Laparoscopic management of adnexal masses. Obstet Gynecol Clin North Am. 2011;38(4):663-76. [PubMed ID: 22134015]. https://doi.org/10.1016/j.ogc.2011.09.003.
-
8.
Oge T, Ozturk E, Yalcin OT. Does size matter? Retrospective analysis of large gynecologic tumors. J Turk Ger Gynecol Assoc. 2017;18(4):195-9. [PubMed ID: 29278233]. [PubMed Central ID: PMC5776159]. https://doi.org/10.4274/jtgga.2017.0022.
-
9.
Demir RH, Marchand GJ. Adnexal masses suspected to be benign treated with laparoscopy. JSLS. 2012;16(1):71-84. [PubMed ID: 22906334]. [PubMed Central ID: PMC3407461]. https://doi.org/10.4293/108680812X13291597716069.
-
10.
Yerebasmaz N, Dilbaz B, Altinbas S, Sengul Ö, Dede FS, Altinbas S. Laparoscopy or laparotomy for large and benign adnexal masses? J Clin Anal Med. 2016;7(3):380-3. https://doi.org/10.4328/jcam.4178.
-
11.
Machida H, Koyasu Y, Yamada M, Nishio M, Yamamoto K. Does tumor size limit application of laparoscopic surgery to ovarian tumors? Gynecology and Minimally Invasive Therapy. 2016;5(4):156-60. https://doi.org/10.1016/j.gmit.2015.03.004.
-
12.
Lim S, Lee KB, Chon SJ, Park CY. Is tumor size the limiting factor in a laparoscopic management for large ovarian cysts? Arch Gynecol Obstet. 2012;286(5):1227-32. [PubMed ID: 22791381]. https://doi.org/10.1007/s00404-012-2445-9.
-
13.
Vlahos NF, Iavazzo C, Marcopoulos MC, Alamanou A, Kouiroukidou P, Chatzidakis V, et al. Laparoscopic management of large ovarian cysts. Surg Innov. 2012;19(4):370-4. [PubMed ID: 22371368]. https://doi.org/10.1177/1553350611432722.
-
14.
Shiota M, Kotani Y, Umemoto M, Tobiume T, Hoshiai H. Study of the correlation between tumor size and cyst rupture in laparotomy and laparoscopy for benign ovarian tumor: Is 10 cm the limit for laparoscopy? J Obstet Gynaecol Res. 2012;38(3):531-4. [PubMed ID: 22353442]. https://doi.org/10.1111/j.1447-0756.2011.01748.x.
-
15.
Detorakis S, Vlachos D, Athanasiou S, Grigoriadis T, Domali A, Chatzipapas I, et al. Laparoscopic cystectomy in-a-bag of an intact cyst: Is it feasible and spillage-free after all? Minim Invasive Surg. 2016;2016:8640871. [PubMed ID: 27099793]. [PubMed Central ID: PMC4821967]. https://doi.org/10.1155/2016/8640871.
-
16.
Bogani G, Cromi A, Serati M, Di Naro E, Casarin J, Pinelli C, et al. Laparoscopic and open abdominal staging for early-stage ovarian cancer: Our experience, systematic review, and meta-analysis of comparative studies. Int J Gynecol Cancer. 2014;24(7):1241-9. [PubMed ID: 25054448]. https://doi.org/10.1097/IGC.0000000000000214.
-
17.
Grammatikakis I, Trompoukis P, Zervoudis S, Mavrelos C, Economides P, Tziortzioti V, et al. Laparoscopic treatment of 1522 adnexal masses: An 8-year experience. Diagn Ther Endosc. 2015;2015:979162. [PubMed ID: 25762854]. [PubMed Central ID: PMC4339861]. https://doi.org/10.1155/2015/979162.
-
18.
Makhija A, Parekh CD, Mankad MH, Desai AD, Dave PS, Patel SM. Rationale of laparoscopic surgery in gynaecological oncology: Time to address the issue!. Indian J Gynecol Oncol. 2018;16(3). https://doi.org/10.1007/s40944-018-0219-4.
-
19.
Falcetta FS, Lawrie TA, Medeiros LR, da Rosa MI, Edelweiss MI, Stein AT, et al. Laparoscopy versus laparotomy for FIGO stage I ovarian cancer. Cochrane Database Syst Rev. 2016;10. CD005344. [PubMed ID: 27737492]. [PubMed Central ID: PMC6464147]. https://doi.org/10.1002/14651858.CD005344.pub4.
-
20.
Gad MS, El Khouly NI, Soto E, Brodman M, Chuang L, Nezhat FR, et al. Differences in perioperative outcomes after laparoscopic management of benign and malignant adnexal masses. J Gynecol Oncol. 2011;22(1):18-24. [PubMed ID: 21607091]. [PubMed Central ID: PMC3097329]. https://doi.org/10.3802/jgo.2011.22.1.18.
-
21.
Ye P, Zhao N, Shu J, Shen H, Wang Y, Chen L, et al. Laparoscopy versus open surgery for adnexal masses in pregnancy: A meta-analytic review. Arch Gynecol Obstet. 2019;299(3):625-34. [PubMed ID: 30706184]. [PubMed Central ID: PMC6394438]. https://doi.org/10.1007/s00404-018-05039-y.
-
22.
Eltabbakh G. Laparoscopic surgery for large ovarian cysts-review. Trends Gynecol Oncol. 2017;2:109. https://doi.org/10.4172/2161-0932.s5:09.
-
23.
Covens AL, Dodge JE, Lacchetti C, Elit LM, Le T, Devries-Aboud M, et al. Surgical management of a suspicious adnexal mass: A systematic review. Gynecol Oncol. 2012;126(1):149-56. [PubMed ID: 22522189]. https://doi.org/10.1016/j.ygyno.2012.04.018.
-
24.
Tantitamit T, Lee CL. Is it the time for laparoscopic management of early-stage ovarian malignancies? Gynecol Minim Invasive Ther. 2018;7(3):93-103. [PubMed ID: 30254949]. [PubMed Central ID: PMC6135162]. https://doi.org/10.4103/GMIT.GMIT_59_18.