Abstract
Background:
Hepatitis C virus (HCV) is the main cause of acute and chronic liver failure. It seems that interleukin-38 (IL-38), a member of IL-1 cytokine family, has an anti-inflammatory function through binding to the receptor of several cytokines.Objectives:
To examine serum level of IL-38 in HCV-infected patients and its correlation with liver enzymes.Methods:
We measured serum levels of IL-38 and liver enzymes in 26 untreated and 11 treated HCV-infected patients and 26 healthy individuals using ELISA and biochemistry assay kits, respectively.Results:
Serum level of IL-38 was significantly lower in untreated HCV+ patients compared to treated patients (P = 0.014) and control group (P < 0.0001). In addition, IL-38 levels negatively correlated with alkaline phosphatase (ALP) levels in untreated HCV+ patients (r = -0.905, P = 0.042). However, no significant correlations were detected between serum levels of IL-38 and other liver enzymes.Conclusions:
Our results suggest a potential anti-inflammatory role for IL-38 in chronic HCV infection which can be implemented to prevent severe hepatic failure in patients with hepatitis C.Keywords
Hepatitis C Virus IL-38 Anti-inflammatory Liver Enzymes Alkaline Phosphatase (ALP)
1. Background
Hepatitis C virus (HCV), a hepatotropic and non-cytopathic RNA virus of the Flaviviridae family, is considered one of the major causes of liver diseases, such as acute and consequently chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma (HCC) (1, 2). World Health Organization (WHO) has recently estimated that 58 million people live with chronic hepatitis C virus infection worldwide, and approximately 290,000 deaths from hepatitis C, mostly from cirrhosis and HCC, occurred in 2019 (3). In HCV infection, both innate and adaptive arms of the immune system are engaged in the activation of antiviral immunity, and defects in each part of the immune system can lead to persistent virus replication in hepatocytes, which participates in the activation of inflammatory cells in the liver and promotes progressive liver fibrosis and damage (4). Recognition of HCV RNA by the cytosolic RNA-binding proteins initiates the activation of antiviral responses (5). Interferons, natural killer (NK) cells, NKT cells, dendritic cells (DCs), monocytes, and macrophages from the innate immune system, along with both CD4+ and CD8+ T cell subsets and T cells’ immunoregulatory cytokines from the adaptive immune response play crucial roles in defense against HCV infection (5-7). It has been proved that during viral infection, cytokines, as intercellular soluble proteins secreted by both innate and acquired immune/non-immune cells, contribute to viral clearance (8). In the acute phase of HCV infection, several pro-inflammatory cytokines such as interleukin-1 (IL-1), IL-8, antiviral type 1 interferon (IFN)-alpha/beta, IFN-gamma, and IL-12 play important roles in the inhibition of viral replication (8, 9). However, it has been observed that HCV can impair the balance of circulating pro-and anti-inflammatory cytokines and chemokines, and cause chronic infection, which triggers liver damage, hepatitis, and cirrhosis (10).
Interleukin-38 (IL-1F10) has been characterized as a member of IL-1 superfamily and shares certain constructional characteristics with IL-1 receptor antagonist (IL-1Ra) (41% homology) and IL-36Ra (43% homology) as well. Hence, IL-38 can bind to the IL-36R and exert anti-inflammatory function by preventing the binding of IL-36R to agonist ligands and reducing inflammation (11, 12). Amplification of multi-tissue cDNA using polymerase chain reaction (PCR) method disclosed the mRNA expression of IL-38 in a wide range of normal tissues, including fetal liver, spleen, thymus, heart, placenta, and particularly in the skin and tonsillar proliferating B cells (12). Besides, association of IL-38 polymorphisms with susceptibility to psoriatic arthritis, rheumatoid arthritis, human cardiovascular disease, and ankylosing spondylitis has been reported in previous studies (13, 14). A study was done on patients with systemic lupus erythematosus showed that IL-38 had protective anti-inflammatory activities, and silencing endogenous IL-38 in PBMCs resulted in a noticeable rise in the expression of pro-inflammatory mediators IL-6, chemokine (C-C motif) ligand 2 (CCL2), and a proliferation-inducing ligand (APRIL) (15). However, the expression pattern and functional features of IL-38 in viral hepatitis, particularly HCV infection, remain vague and need to be explored in further research.
The conventional treatment landscape of HCV infection has been revolutionized with the advent of an oral direct-acting antiviral (DAA) regimen with a high rate of sustained virologic response (SVR) and short treatment duration (16). Sofosbuvir (SOF) and daclatasvir (DCV) possess some inhibitory features against HCVNS5B polymerase and NS5A protein of HCV, respectively, and are utilized as Food and Drug Administration (FDA)-approved treatment for HCV-infected patients with 1 and 3 genotypes. Results from clinical practice of the SOF and DCV combined regimen in the treatment of Iranian with hepatitis C demonstrated a 98% SVR rate in both 1 and 3 genotypes (17). Analysis of the treatment outcomes from HCV-infected patients can help us to find the mechanism of viral-host interactions. To increase our knowledge about serum level of IL-38 in HCV-infected patients and recognize the effects of therapeutic regimen, we evaluated serum IL-38 level in HCV-infected patients before and after treatment with DAAs along with healthy subjects, as well as identified the correlation of IL-38 level with liver enzymes levels, including aspartate transaminase (AST), alanine transaminase (ALT), and alkaline phosphatase (ALP).
2. Methods
2.1. Subjects
Twenty-six newly-diagnosed patients with HCV infection (25 men and 1 woman) along with 26 age- and sex- matched healthy subjects (25 men and 1 woman) were recruited in the present study from Motahari Clinic affiliated to Shiraz University of Medical Sciences, Shiraz, Iran, from September to October 2019. All patients were checked to be positive for HCV by detecting HCV-RNA (real-time PCR) and anti-HCV antibodies (EIAgen HCV Ab, Adaltis, Italy). HCV-infected patients were then treated with a pangenotypic antiviral regimen comprising sofosbuvir 400 mg/day and daclatasvir 60 mg/day for 12 weeks (n = 11). The efficacy of treatment was assessed by detection of viral RNA in patients’ sera. Participants who received antiviral drugs or had a history of non-alcoholic fatty liver disease (NAFLD), non-alcoholic steatohepatitis (NASH), cholestatic liver diseases, cardiac cirrhosis, primary biliary cirrhosis, and immunodeficiency states were excluded from the study. The clinical characteristics of enrolled cases, including age and gender, as well as the serum levels of AST, ALT, and ALP, are shown in Table 1.
| Untreated HCV Patients (n = 26) | Treated HCV Patients (n = 11) | Healthy Group (n = 26) | |
|---|---|---|---|
| Age (y) | 42.26 ± 9.8 | 41.6 ± 9.3 | 42/20 ± 10 |
| Sex | |||
| Male | 25 | 11 | 25 |
| Female | 1 | 0 | 1 |
| ALT (U/L) | 37.79 ± 30.3 | 49.9 ± 54.4 | 17 ± 12.4 |
| AST (U/L) | 39.41 ± 28 | 34.5 ± 11.8 | 24 ± 9.1 |
| ALP (U/L) | 180.13 ± 61.2 | 166.5 ± 73.9 | 157.2 ± 22 |
2.2. Sampling
5 mL of peripheral blood was collected from healthy controls (n = 26) and HCV-infected patients before (n = 26) and after (n = 11) receiving antiviral therapy by venipuncture. After resting for 30 min at room temperature (RT), samples were centrifuged at 3,000 × g for 10 min, and the sera were separated and stored at -70°C until use.
2.3. Ethics Statement
The study was ethically approved by Medical Ethics Committee of Shiraz University of Medical Sciences (IR.sums.med.rec.1399.81, link: ethics.research.ac.ir/ProposalCertificate.php?id=133659) and written informed consent was obtained from each participant according to the Declaration of Helsinki.
2.4. Serological Analysis of Interleukin-38, Aspartate Transaminase, Alanine Transaminase, and Alkaline Phosphatase
The serum levels of IL-38 in subjects were measured using Enzyme-linked Immunosorbent Assay (ELISA) kit (Biohippo Inc, Gaithersburg, United States of America) following by manufacturer’s instructions. Briefly, 100 μL of standard and sample was added to each well of 96-well ELISA plate and incubated for two hours at 25 ± 2°C. After washing, the plate was incubated with 100 μL Biotin-Conjugate antibody for 1 hour at RT. Afterward, the plates were washed, and 100 μL/well of Streptavidin-HRP working solution was added. After incubating for 30 minutes and three washes of each well, 100 μL TMB substrate was added, and the plate was incubated in the dark place for 15 minutes at RT. Finally, 50 μL stop solution was added to each well, and the optical density of samples was read using a microplate reader (R & D, USA) at 450 nm. IL-38 concentration was determined using a curve created based on standards optical density. The minimum and maximum detectable dose of IL-38 in this assay were 15 ng/L and 4,000 ng/L, respectively.
Regarding liver enzymes, serum levels of ALT, AST, and ALP were measured by Pars Azmoon reagents kits (Iran) using automatic biochemistry analyzer (Roche Hitachi 912, Japan).
2.5. Statistical Analysis
Data were analyzed using the GraphPad Prism version 6.0 (GraphPad Software, Inc., CA, US) and expressed as mean ± SEM. P-values < 0.05 was considered statistically significant. Comparisons between two groups and correlations were performed using student t-test and Pearson’s correlation test, respectively.
3. Results
3.1. Serum Interleukin-38 Levels in Untreated and Treated Hepatitis C Virus-Infected Patients Compared to Healthy Controls
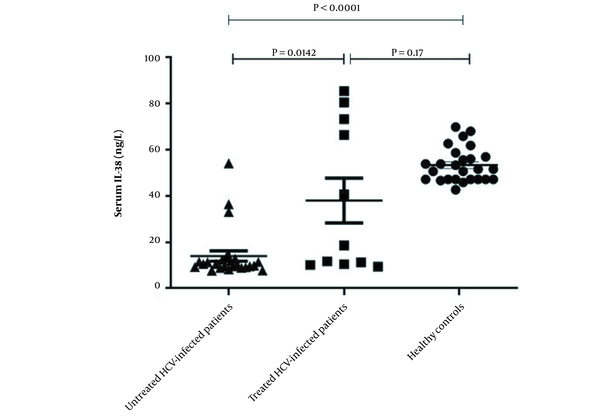
ELISA was used to investigate level of IL-38 in the serum of enrolled participants. As displayed in Figure 1, the serum level of IL-38 was significantly lower in untreated HCV patients compared to those who were treated with a combination of sofosbuvir and daclatasvir (11.22 ± 1.1 vs. 37.95 ± 9.64, P = 0.014) and the healthy individuals as well (11.22 ± 1.1 vs. 53.26 ± 7.37, P < 0.0001). However, there was no significant difference between serum IL-38 levels of treated HCV patients and control group (37.95 ± 9.64 vs. 53.26 ± 7.37, P = 0.17) (Figure 1).
Serum levels of interleukin-38 in hepatitis C virus-infected patients before and after treatment and healthy group. Serum levels of IL-38 in untreated patients (n = 26), treated patients (n = 11) and healthy individuals (n = 26) were measured using ELISA. The level of IL-38 in the serum of HCV-infected patients before treatment was lower than the post-treated patients and the control group. No significant difference in serum level of IL-38 was detected between post-treated patients and healthy controls. Data are presented as mean ± SEM and P-value < 0.05 was considered statistically significant.
3.2. Relationship Between Serum Interleukin-38 and Liver Enzymes Levels
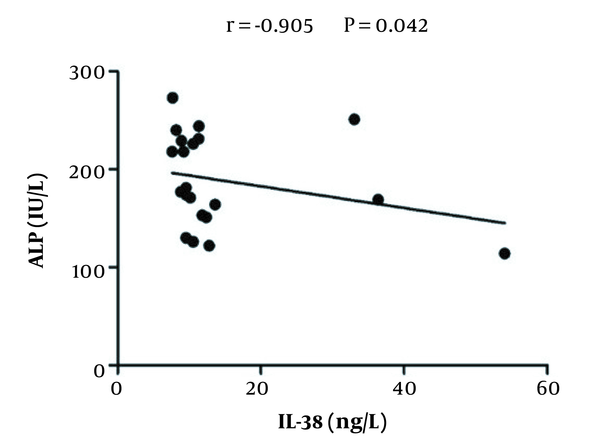
To explore whether the IL-38 serum level correlated to the changes of liver enzymes level, Pearson’s correlation analysis was applied. As is shown in Figure 2, serum IL-38 levels negatively correlated with serum ALP levels (r = -0.905, P = 0.042) in HCV-infected patients prior to treatment; however, no significant correlations were detected between levels of IL-38 and other enzymes (AST and ALT) (data not shown). Similarly, there were no significant correlations between levels of IL-38 and liver enzymes in the serum of treated patients and healthy controls (data not shown).
Pearson correlation analysis of the interleukin-38 levels with ALP levels in hepatitis C virus-infected patients prior to treatment. There was a negative correlation between IL-38 and alkaline phosphatase levels in untreated patients.
4. Discussion
Emerging evidence supports that cytokines play a key role in the elimination of viruses and the induction of protective immunity. The balance between pro-and anti-inflammatory responses is very important during the course of HCV infection; so, disturbing this balance leads to HCV persistence and eventually liver damage (18, 19). Therefore, the knowledge about the production and secretion of different cytokines during acute and chronic phases of HCV infection can contribute to the identification of helpful immunotherapeutic strategies. Several studies have shown some variations in serum pro- and anti-inflammatory cytokine levels, such as IL-2, IL-4, IL-10, IL-6, IL-8, TNF-α, IL-12, IL-35 and IFN-gamma during HCV infection (2, 20-23). Our previous studies reported a significant increase in IL-18 and IL-35 serum levels in HCV-infected patients (24, 25).
IL-38, a new member of IL-1 family, is recognized by immunosuppressive function in the inflammatory responses by binding to IL-1 family receptors (IL-1R, IL-36R). Van de Veerdonk et al. reported that the addition of IL-38 to the peripheral blood mononuclear cells culture, reduced the production of IL-17A, IL-22, and IL-8 under inflammatory conditions; however, at high concentrations, IL-38 elevated the IL-17 and IL-22 levels (11). The anti-inflammatory features of IL-38 have been reported in allergic asthma as well as autoimmune diseases like SLE and rheumatoid arthritis (15, 26, 27). These findings indicate that IL-38 is linked with the immunopathogenesis of chronic inflammatory diseases. However, the role of IL-38 during the course HCV infection has not been elucidated yet. The present study aimed to evaluate serum level of IL-38 in HCV-infected patients and recognize the effects of therapeutic regimen. For these purposes, we measured serum IL-38 level in HCV-infected patients before and after treatment with DAAs, and healthy individuals. Our results indicated that the levels of serum IL-38 were significantly lower in untreated HCV-infected patients as compared to the control group (P < 0.0001) and this may be due to the predominance of inflammatory responses in the early phase of HCV infection. After treatment with sofosbuvir and daclatasvir, however, the serum IL-38 levels increased remarkably in the treated group (P = 0.014), which indicated a relationship between IL-38 and HCV viral replication. By contrast, Wang et al. examined the serum levels of IL-38 in patients with chronic hepatitis B (CHB) and revealed IL-38 levels in untreated CHB patients with existing liver injury were higher than in healthy controls and returned to normal after treatment with telbivudine for 12 weeks. Moreover, a positive correlation has been found between the serum levels of IL-38 and aspartate aminotransferase in cases with CHB, which indicated that IL-38 level may be a marker reflecting liver injury (14). In the present study, however, correlation analysis of serum levels of IL-38 with AST and ALT demonstrated that serum IL-38 level were not associated with serum AST and ALT levels in all groups (data not shown), but a negative correlation was found between serum IL-38 and ALP levels in untreated HCV+ patients. As damaged liver cells release increased the levels of AST, ALT, and ALP, monitoring serum AST, ALT, and ALP levels can indicate the grade or stage of liver damage and fibrosis (28). Based on our findings, it seems that IL-38 inhibits the progression of chronic hepatitis. In 2016 Mora et al. demonstrated that the IL-38 released by apoptotic cells could limit inflammation (29). They found that apoptotic cells increased the expression of IL-38 mRNA and released high amounts of IL-38, which suppressed the production of IL-6 and IL-8 by macrophages. It has also been reported that macrophages cultured in apoptotic cell-conditioned medium reduced the production of IFN-γ, IL-17, and IL-10 by T cells. As the presence of IL-6 is necessary for Th17 differentiation and expansion, suppressed IL-6 production by IL-38 can explain this reduction (29).
It must be considered that this study measured the serum IL-38 level of HCV-infected patients for the first time, so further research is necessary to focus on the cardinal mechanisms exerted by the IL-38 in both innate and adaptive immune responses against HCV in the large-scale population surveys which may be a valuable weapon for preventing the hepatic severe damage and failure in patients with hepatitis C. One of the limitations of this study was the absence of measured levels of inflammatory cytokines such as IL-6, IL-1β, and TNF-α and their correlations with IL-38 levels among untreated, treated HCV-infected patients and healthy individuals.
In conclusion, our results indicated that the levels of serum IL-38 in untreated HCV-infected patients were lower than those who were treated with SOF/DCV for 12 weeks as well as healthy subjects. In addition, there was a negative correlation between IL-38 and ALP levels in untreated patients with hepatitis C. Thus, we propose that IL-38 can be implemented as a prognostic biomarker for assessing the severity of disease in chronic HCV infection and designing an appropriate treatment choice.
References
-
1.
Wieland SF, Chisari FV. Stealth and cunning: hepatitis B and hepatitis C viruses. J Virol. 2005;79(15):9369-80. [PubMed ID: 16014900]. [PubMed Central ID: PMC1181548]. https://doi.org/10.1128/JVI.79.15.9369-9380.2005.
-
2.
Liu S, Zhang Q, Shao X, Wang W, Zhang C, Jin Z. An immunosuppressive function of interleukin-35 in chronic hepatitis C virus infection. Int Immunopharmacol. 2017;50:87-94. [PubMed ID: 28644966]. https://doi.org/10.1016/j.intimp.2017.06.015.
-
3.
World Health Organization. Hepatitis C Fact Sheet. Geneva, Switzerland: World Health Organization; 2022, [updated 24 June 2022; cited 27 July 2021]. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c.
-
4.
Li H, Huang MH, Jiang JD, Peng ZG. Hepatitis C: From inflammatory pathogenesis to anti-inflammatory/hepatoprotective therapy. World J Gastroenterol. 2018;24(47):5297-311. [PubMed ID: 30598575]. [PubMed Central ID: PMC6305530]. https://doi.org/10.3748/wjg.v24.i47.5297.
-
5.
Dustin LB. Innate and Adaptive Immune Responses in Chronic HCV Infection. Curr Drug Targets. 2017;18(7):826-43. [PubMed ID: 26302811]. [PubMed Central ID: PMC5625838]. https://doi.org/10.2174/1389450116666150825110532.
-
6.
Park SH, Rehermann B. Immune responses to HCV and other hepatitis viruses. Immunity. 2014;40(1):13-24. [PubMed ID: 24439265]. [PubMed Central ID: PMC4480226]. https://doi.org/10.1016/j.immuni.2013.12.010.
-
7.
Heim MH, Thimme R. Innate and adaptive immune responses in HCV infections. J Hepatol. 2014;61(1 Suppl):S14-25. [PubMed ID: 25443342]. https://doi.org/10.1016/j.jhep.2014.06.035.
-
8.
Fallahi P, Ferri C, Ferrari SM, Corrado A, Sansonno D, Antonelli A. Cytokines and HCV-related disorders. Clin Dev Immunol. 2012;2012:468107. [PubMed ID: 22611419]. [PubMed Central ID: PMC3352261]. https://doi.org/10.1155/2012/468107.
-
9.
Tawfik AK, Amin AM, Yousef M, El-Sayd NM, Elashry H, Elkadeem M, et al. IL-1alpha correlates with severity of hepatitis C virus-related liver diseases. J Inflamm Res. 2018;11:289-95. [PubMed ID: 30022847]. [PubMed Central ID: PMC6044361]. https://doi.org/10.2147/JIR.S166564.
-
10.
Qian S, Golubnitschaja O, Zhan X. Chronic inflammation: key player and biomarker-set to predict and prevent cancer development and progression based on individualized patient profiles. EPMA J. 2019;10(4):365-81. [PubMed ID: 31832112]. [PubMed Central ID: PMC6882964]. https://doi.org/10.1007/s13167-019-00194-x.
-
11.
van de Veerdonk FL, Stoeckman AK, Wu G, Boeckermann AN, Azam T, Netea MG, et al. IL-38 binds to the IL-36 receptor and has biological effects on immune cells similar to IL-36 receptor antagonist. Proc Natl Acad Sci U S A. 2012;109(8):3001-5. [PubMed ID: 22315422]. [PubMed Central ID: PMC3286950]. https://doi.org/10.1073/pnas.1121534109.
-
12.
Yuan X, Peng X, Li Y, Li M. Role of IL-38 and its related cytokines in inflammation. Mediators Inflamm. 2015;2015:807976. [PubMed ID: 25873772]. [PubMed Central ID: PMC4383490]. https://doi.org/10.1155/2015/807976.
-
13.
van de Veerdonk FL, Netea MG. New Insights in the Immunobiology of IL-1 Family Members. Front Immunol. 2013;4:167. [PubMed ID: 23847614]. [PubMed Central ID: PMC3703542]. https://doi.org/10.3389/fimmu.2013.00167.
-
14.
Wang HJ, Jiang YF, Wang XR, Zhang ML, Gao PJ. Elevated serum interleukin-38 level at baseline predicts virological response in telbivudine-treated patients with chronic hepatitis B. World J Gastroenterol. 2016;22(18):4529-37. [PubMed ID: 27182162]. [PubMed Central ID: PMC4858634]. https://doi.org/10.3748/wjg.v22.i18.4529.
-
15.
Rudloff I, Godsell J, Nold-Petry CA, Harris J, Hoi A, Morand EF, et al. Brief Report: Interleukin-38 Exerts Antiinflammatory Functions and Is Associated With Disease Activity in Systemic Lupus Erythematosus. Arthritis Rheumatol. 2015;67(12):3219-25. [PubMed ID: 26314375]. https://doi.org/10.1002/art.39328.
-
16.
Kish T, Aziz A, Sorio M. Hepatitis C in a New Era: A Review of Current Therapies. P t. 2017;42(5):316-29. eng. [PubMed ID: 28479841]. [PubMed Central ID: PMC5398625].
-
17.
Alavian SM, Hajarizadeh B, Bagheri Lankarani K, Sharafi H, Ebrahimi Daryani N, Merat S, et al. Recommendations for the Clinical Management of Hepatitis C in Iran: A Consensus-Based National Guideline. Hepat Mon. 2016;16(8). e40959. [PubMed ID: 27799966]. [PubMed Central ID: PMC5075356]. https://doi.org/10.5812/hepatmon.guideline.
-
18.
Tsai SL, Sheen IS, Chien RN, Chu CM, Huang HC, Chuang YL, et al. Activation of Th1 immunity is a common immune mechanism for the successful treatment of hepatitis B and C: tetramer assay and therapeutic implications. J Biomed Sci. 2003;10(1):120-35. [PubMed ID: 12566993]. https://doi.org/10.1007/BF02256004.
-
19.
Wan L, Kung YJ, Lin YJ, Liao CC, Sheu JJ, Tsai Y, et al. Th1 and Th2 cytokines are elevated in HCV-infected SVR(-) patients treated with interferon-alpha. Biochem Biophys Res Commun. 2009;379(4):855-60. [PubMed ID: 19118522]. https://doi.org/10.1016/j.bbrc.2008.12.114.
-
20.
Fan X, Liu W, Li C. [Determination of serum cytokines in individuals with HCV infection]. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2000;14(2):145-7. [PubMed ID: 11503046].
-
21.
Spanakis NE, Garinis GA, Alexopoulos EC, Patrinos GP, Menounos PG, Sklavounou A, et al. Cytokine serum levels in patients with chronic HCV infection. J Clin Lab Anal. 2002;16(1):40-6. [PubMed ID: 11835530]. [PubMed Central ID: PMC6808060]. https://doi.org/10.1002/jcla.2060.
-
22.
Sofian M, Aghakhani A, Farazi AA, Banifazl M, Eslamifar A, Rashidi N, et al. Serum profile of T helper 1 and T helper 2 cytokines in hepatitis C virus infected patients. Hepat Mon. 2012;12(12). e6156. [PubMed ID: 23423691]. [PubMed Central ID: PMC3575547]. https://doi.org/10.5812/hepatmon.6156.
-
23.
Araujo AR, Peruhype-Magalhaes V, Coelho-dos-Reis JG, Chaves LP, de Lima TA, Pimentel JP, et al. Dual role of IL-12 in the therapeutic efficacy or failure during combined PEG-Interferon-alpha2A and ribavirin therapy in patients with chronic hepatitis C. Immunol Lett. 2013;154(1-2):61-9. [PubMed ID: 23973662]. https://doi.org/10.1016/j.imlet.2013.07.010.
-
24.
Asadipour M, Fazeli P, Zohouri M, Bemani P, Mohebbiniya M, Khansalar S, et al. IL-18 in Blood Serum of Hepatitis C Patients Might be of Predictive Value for Individual Outcomes. Infect Disord Drug Targets. 2021;21(3):389-93. [PubMed ID: 32634083]. https://doi.org/10.2174/1871526520666200707113401.
-
25.
Taghinejad A, Barani S, Gholijani N, Ghandehari F, Khansalar S, Asadipour M, et al. Variations in IL-22, IL-27 and IL-35 serum levels in untreated and treated hepatitis C patients. Eur Cytokine Netw. 2020;31(4):134-9. [PubMed ID: 33648921]. https://doi.org/10.1684/ecn.2020.0455.
-
26.
Takenaka SI, Kaieda S, Kawayama T, Matsuoka M, Kaku Y, Kinoshita T, et al. IL-38: A new factor in rheumatoid arthritis. Biochem Biophys Rep. 2015;4:386-91. [PubMed ID: 29124228]. [PubMed Central ID: PMC5669445]. https://doi.org/10.1016/j.bbrep.2015.10.015.
-
27.
Sun X, Hou T, Cheung E, Iu TN, Tam VW, Chu IM, et al. Anti-inflammatory mechanisms of the novel cytokine interleukin-38 in allergic asthma. Cell Mol Immunol. 2020;17(6):631-46. [PubMed ID: 31645649]. [PubMed Central ID: PMC7264207]. https://doi.org/10.1038/s41423-019-0300-7.
-
28.
Giannini EG, Testa R, Savarino V. Liver enzyme alteration: a guide for clinicians. CMAJ. 2005;172(3):367-79. [PubMed ID: 15684121]. [PubMed Central ID: PMC545762]. https://doi.org/10.1503/cmaj.1040752.
-
29.
Mora J, Schlemmer A, Wittig I, Richter F, Putyrski M, Frank AC, et al. Interleukin-38 is released from apoptotic cells to limit inflammatory macrophage responses. J Mol Cell Biol. 2016;8(5):426-38. [PubMed ID: 26892022]. https://doi.org/10.1093/jmcb/mjw006.