Abstract
Background:
In the recent years, the focus of consumers to less popular fruits such as Cornus mas, with unusual flavor as well as rich antioxidant and anthocyanins content has increased. C. mas fruits have been used for treatment of gastrointestinal disorders.Objectives:
The aim of this study was to investigate the hepatoprotective effect of C. mas fruits extract (CMFE) against carbon tetrachloride-induced hepatic damage in male albino rats.Materials and Methods:
Hepatotoxicity was induced by administration of CCl4 (1 mL/kg i.p. (Intraperitoneal)) in olive oil with 1:1 dilution ratio. To evaluate the effect of CMFE on the disease progression, serum marker enzymes, serum total protein and albumin, liver lipid peroxidation and antioxidant enzymes activities were determined in CCl4-induced hepatotoxicity.Results:
Oral administration of CMFE to rats for 16 days, afforded significant (P < 0.05) hepatoprotection against CCl4-induced elevation in serum marker enzymes activities, serum total protein and albumin, and liver lipid peroxidation, as well as significant (P < 0.05) reduction in liver antioxidant enzymes activities, such as superoxide dismutase, catalase and glutathione peroxidase activities.Conclusions:
The present study indicated that the beneficial effect of C. mas extract might be due to the presence of some antioxidant components with membrane-stabilizing effects.Keywords
1. Background
Hepatic injuries lead to attenuation of metabolic functions regulated by liver and remain as one of the serious health problems (1) threatening the health of human societies. The pathogenesis of liver fibrosis is not clear, reactive oxygen species (ROS) have important roles in liver pathological changes (2). Unsaturated fatty acids of biological cell membranes (sensitive parts of cells against free radicals), get affected by the peroxidation reaction, which decreases the fluidity and disruption of membrane function and integrity, leading to serious pathological changes (3).
Biotransformation of carbon tetrachloride (CCl4) to trichloromethyl free radicals (CCl3 or CCl3OO) by hepatic microsomal cytochrome P450, is a well-known model compound causing chemical hepatic injury (4-7). When the ROS forms are highly produced, additional protective mechanisms of dietary antioxidants may be of a great importance (8, 9). Antioxidant nutrients and enzymes are the cooperative protective defense systems against free radicals damages (10). Antioxidants and radical scavengers have been studied on the mechanism of CCl4 toxicity. They protect the liver cells from the CCl4-induced damage due to lipid peroxidation (11).
Natural antioxidants such as fruits and vegetables, which provide protection against free radicals, can decrease the incidence and mortality rates of cancer and heart diseases, in addition to their other health benefits (12). Recently, the consumption of herbs such as cornelian cherry (Cornus mas), with high levels of antioxidants and anthocyanins, has been increased. C. mas fruits are prescribed for gastrointestinal and excretory disorders (13), beside improving liver and kidney functions (14, 15). This plant was used to treat diarrhea, intestinal inflammation, fever, malaria, kidney stones and kidney and bladder infections in traditional medicine. C. mas fruits has anthocyanins, flavonoids, and plenty of oxalic acid content (16). It also contains antioxidant substances including butylated hydroxyanisole and butylated hydroxytoluene, and has the potential to fight cancer (17, 18).
2. Objectives
This study was designed to investigate the protective effects of C. mas fruits extract (CMFE) against CCl4-induced hepatotoxicity in rats.
3. Materials and Methods
3.1. Chemicals
Ethylene diamine tetra acetic acid (EDTA) and trichloroacetic acid (TCA) were obtained from Sigma-Aldrich Chemical Co. Ltd. (USA). CCl4 and thiobarbituric acid (TBA) were obtained from Merck Co. (Germany).
3.2. Plant Materials
C. mas fruits were obtained from suburbs of Kaleibar (East Azarbayjan, Iran) at the end of summer 2011. The fruits were air-dried and then ground into powder; in all the steps, the components were protected from direct sunlight. The powder was kept at 8°C.
3.3. Extraction
The air-dried C. mas fruits were ground into a coarse powder, 500 g of which was mixed with a methanol:water (7:3) solution at 25 ± 2°C. The solvent was completely removed by rotary vacuum evaporator at 50°C. Afterwards, CMFE was frozen at -20°C until use.
3.4. Animals and Treatment
The animals (Wistar strain male albino rats [250 - 300 g]) were kept in polypropylene cages in a room with 22 ± 2°C temperature, humidity of 44-55%, and light and dark cycles (12/12 hours for each), for 1 week before and during the experiments. Animals were fed with a standard rodent pellet diet and clean drinking water ad libitum. The protocol of this study was approved by Animal Ethic Committee of Tabriz University of Medical Sciences (AECTUMS).
Animals were divided to six groups (N = 6), as follows:
Group I: normal controls, received drinking water orally for 16 days, and olive oil on the 16th day (1 mL/kg i.p.).
Group II: toxic controls, received drinking water for 16 days orally, and CCl4 (1 mL/kg i.p.) in 1:1 dilution with olive oil on the 16th day.
Group III and IV: pretreatment groups, received CMFE orally with doses of 200 and 500 mg/kg for 16 days, and CCl4 on the 16th day (1 mL/kg i.p.), 2 hours after administration of the last dose of extract.
Group V and VI: post-treatment groups, received drinking water orally for 16 days, and CCl4 (1 mL/kg i.p.) on the 16th day at a ratio similar to the above groups, followed by CMFE at doses of 200 mg/kg (Group V) and 500 mg/kg (Group VI) orally at 2, 6, 12, 24 and 48 hours after CCl4 toxification.
3.5. Provision of Liver Homogenate
Fifty hours after the CCl4 administration animals were sacrificed. The hepatic tissues were homogenized in KCl [10 mM] phosphate buffer (1.15%) with EDTA (pH = 7.4) and centrifuged at 3000 rpm for 30 minutes. The supernatant was collected to be used for measurement of malondialdehyde (MDA), catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPx). The total protein content was determined based on Lowry’s method (19).
3.6. Designation of Lipid Peroxidation
Liver homogenate lipid peroxidation was measured based on formation of thiobarbituric acid reactive substance (TBARS). Of thiobarbituric acid reagent, 2 mL (15% w/v TCA, 0.375% w/v TBA and 0.25 M HCl) was added to 2 mL of the supernatant. The dilution was heated for 15 minutes in boiling water. After cooling, it was centrifuged at 1000 g for 10 minutes and the precipitate was removed. MDA forms were mixed with TBA, which was measured by spectrophotometer at 532 nm. The concentration of MDA was computed based on the absorbance coefficient of the TBA–MDA complex (ε = 1.56 × 105/M/cm), and presented as nmol/mg of protein (20).
3.7. Antioxidant Enzymes Activity
For the measurement of catalase activity, one unit of catalase was required to decompose 1 µM of H2O2 in 1 minute. By adding 1.0 mL of 20 mM H2O2 (freshly prepared), the reaction was inaugurated. Decomposition level of H2O2 was determined by spectrophotometer at 240 nm for 2 minutes. The enzyme activity was presented as U/mg of protein (21).
The SOD activity was assayed, as described by Winterbourn et al. The reaction mixture contained 3 mL of 0.067 M potassium phosphate buffer (pH = 7.8), 0.2 mL of 0.1 M EDTA, 0.3 mM sodium cyanide (NaCN) and 0.1 mL of 1.5 mM nitroblue tetrazolium (NBT). One unit of enzyme activity (as amount of SOD) was determined to produce a 50% inhibition of NBT reduction and the specific enzyme activity was presented as units per milligram of total protein.
The GPx activity was evaluated by Paglia and Valentine method (22). The provided solution containined 400 µL of 0.25 M potassium phosphate buffer (pH = 7.0), 200 µL supernatant, 100 µL GSH (10 mM), 100 µL NADPH (2.5 mM) and 100 µL glutathione reductase (6 U/mL). The reaction start coincided with adding 100 µL hydrogen peroxide (12 mM); the absorbance was measured at 366 nm every 1 minute in a range of 5 minutes (with molar extinction coefficient of 6.22 × 103/M/cm). Data were presented as U/mg of protein.
3.8. Histopathological Studies
After the experiment, the animals were scarified and their livers removed immediately. The liver tissues were fixed in 10% formalin, the paraffin blocks of liver were provided, and then histological sections of 5 - 6 µm in thickness were prepared. The samples were stained with haematoxylin-eosin solution and observed under a light microscope.
3.9. Statistical Analysis
Data were presented as mean ± SE. One way analysis of variance (ANOVA) followed by multiple comparison with the Tukey post-hoc test was used to compared different parameters between the groups. The significance level was considered at P < 0.05.
4. Results
4.1. The Effect of CMFE on Lipid Peroxidation
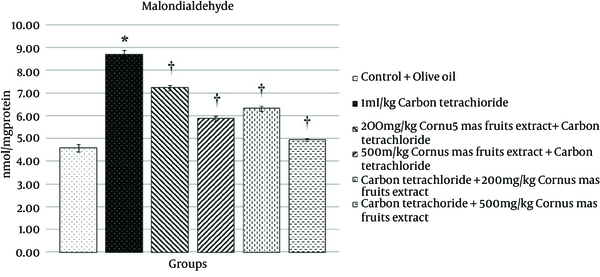
Evaluated level of malondialdehyde content in homogenate of rat liver is shown in Figure 1. MDA contents in the liver homogenate were significantly (P < 0.05) increased in the toxic group compared with the control group. MDA levels of CMFE treatment groups at 200 and 500 mg/kg were significantly (P < 0.05) decreased compared with the toxic group.
| Treatment | Catalase, U/mg Protein | Superoxide Dismutase, U/mg Protein | Glutathione Peroxidase, U/mg Protein |
|---|---|---|---|
| Control + olive oil | 50.37 ± 3.50 | 1.96 ± 0.30 | 5.64 ± 0.16 |
| 1 mL/kg of CCl4 | 10.26 ± 2.42 c | 0.49 ± 0.08 c | 1.68 ± 0.13 c |
| 200 mg/kg of CMFE + CCl4 | 23.21 ± 1.34 d | 0.99 ± 0.04 d | 2.6 ± 0.10 d |
| 500 mg/kg of CMFE + CCl4 | 20.66 ± 0.97 d | 1.18 ± 0.04 d | 3.18 ± 0.07 d |
| CCl4 + 200 mg/kg of CMFE | 29.35 ± 1.34 d | 1.33 ± 0.03 d | 3.65 ± 0.12 d |
| CCl4 + 500 mg/kg of CMFE | 32.48 ± 1.46 d | 1.43 ± 0.03 d | 3.61 ± 0.14 d |
4.2. The Effect of CMFE on Antioxidant Enzymes Activity in the Liver Tissue
The effect of CMFE on SOD, CAT and GPx activities in liver are shown in the Table 1. Antioxidant enzymes activities in the CCl4 group were lower than the normal control. The activities of these enzymes in the CMFE treatment groups significantly (P < 0.05) increased compared with the toxic group.
The Effect of CMFE on the Liver Contents of Malondialdehyde in Rat
4.3. The Effect of CMFE on Histopathology of Liver
Histopatological study of liver on the control group animals showed a normal hepatic architecture with distinct hepatic cells as well as sinusoidal and port spaces. Pathological liver sections of Group II showed drastic changes throughout the lobules, with cellular vacuolization, fatty accumulations and necrosis. Furthermore, the histology report indicated inflammatory infiltrations of the portal triads, dilation of Disse spaces with local disruption of the sinusoidal endothelium and distraction of the central venules. The treated animals with CMFE at 200 and 500 mg/kg (groups III-VI), showed low levels of hepatocellular vacuolation and dilation of Disse spaces, better protection for the normal liver structure in association with moderate hepatocyte plate disorganizations. Treated animals showed rare and in some cases no periportal inflammatory infiltration in the liver lobules.
5. Discussion
Our study showed the hepatoprotective effects of CMFE against liver injury caused by CCl4 in rats. Increased generation of ROS has a major effect on the pathogenesis of related diseases as well as toxicity of a wide range of compounds (23). Nowadays, hepatoprotective drugs against liver damages induced by CCl4 (a hepatotoxic agent) are generally used. Histological signs and features of liver damage caused by CCl4 are similar to acute viral hepatitis. CCl4 has been commonly used in rat experimental models to investigate the oxidative stress, induced in various organs. To the best of our knowledge, this is the first study to evaluate these effects of CMFE in an attempt to prevent liver damage caused by CCl4.
Oxidative stress initiates lipid peroxidation of cell membrane polyunsaturated fatty acids (24). Lipid peroxidation represents one of the most frequent reactions resulting from the free radicals attacks to biological structures as well as accumulation of oxidized lipids in the cell membrane (25). Our results showed the reduction effect of CMFE on TBARS production. In this study, CCl4 administration significantly (P < 0.05) increased the hepatic MDA content, probably revealing the increase of lipid peroxidation. The significant decrease in the hepatic malondialdehyde content, as a marker of lipid peroxidation, confirmed that treatment with CMFE could have a great protective effect against CCl4-induced hepatic lipid peroxidation.
Intracellular ROS concentration is a conclusion of their production and elimination by diverse antioxidants. Major components of the antioxidant system in mammalian cells include SOD, CAT and GPx. These enzymes have important roles in eliminating superoxide anion and H2O2 in cells (26). SOD, an important antioxidant enzyme, catalyzes the highly reactive toxic superoxide radicals to H2O2 (27), and H2O2 decomposition to oxygen and water are catalyzed by catalase and GPx (27, 28). GPx is a glutathione-related enzyme, which can catalyze the synthesis of GST to decrease lipid peroxidation. Combination of free radicals (CCl3) and cell proteins is associated with reaction of sulfhydryl groups of free glutathione and protein thiols, increasing to high serum levels of GST (a phase II enzyme) (29), leading to lipid peroxidation of cell membrane and necrosis (4, 30). Our results indicated that the levels of antioxidant enzymes such as SOD, CAT and GPx, decreased in the CCl4-treated group, were recovered by CMFE treatment. The protective effects of CMFE in maintaining the above enzymes close to the control level increased the capacity of endogenous antioxidant defense as well as their steady state. These effects can also enhance the enzymes synthesis rates, conferring enhanced protection against oxidative stress. Gross necrosis, massive fatty changes, extensive infiltration of lymphocytes, Kupffer cells around the portal vein, and dissociation of cell borders were observed in livers of CCl4 intoxicated rats. The histopathological evaluations of rat livers treated with CMFE, following receiving CCl4, showed prophylactic effect of CMFE on nearly normal structures of liver and toxin-induced hepatic lesions. This protection mechanism provides resistance to liver against toxin-induced damages, through hepatic regeneration stimulation and liver lipid peroxidation inhibition (31).
Antioxidant components of CMFE may cause membrane stabilization and reverse the normalization of fluctuated biochemical profiles induced by CCl4 exposure. Therefore, plant extract compounds affect the liver by maintaining its normal function and decreasing the derangements of cell membrane. Purification of C. mas active components for determining their exact protective effects on hepatocytes is recommended for further studies.
Acknowledgements
References
-
1.
Wolf PL. Biochemical diagnosis of liver disease. Indian J Clin Biochem. 1999;14(1):59-90. [PubMed ID: 23105203]. https://doi.org/10.1007/BF02869152.
-
2.
Poli G, Parola M. Oxidative damage and fibrogenesis. Free Radic Biol Med. 1997;22(1-2):287-305. [PubMed ID: 8958154].
-
3.
Sies H. Strategies of antioxidant defense. Eur J Biochem. 1993;215(2):213-9. [PubMed ID: 7688300].
-
4.
Brattin WJ, Glende EA, Jr, Recknagel RO. Pathological mechanisms in carbon tetrachloride hepatotoxicity. J Free Radic Biol Med. 1985;1(1):27-38. [PubMed ID: 3915301].
-
5.
Rechnagel RO, Glende EA, Jr. Carbon tetrachloride hepatotoxicity: an example of lethal cleavage. CRC Crit Rev Toxicol. 1973;2(3):263-97. [PubMed ID: 4357489]. https://doi.org/10.3109/10408447309082019.
-
6.
Rikans LE, Hornbrook KR, Cai Y. Carbon tetrachloride hepatotoxicity as a function of age in female Fischer 344 rats. Mech Ageing Dev. 1994;76(2-3):89-99. [PubMed ID: 7885069].
-
7.
Shenoy KA, Somayaji SN, Bairy KL. Hepatoprotective effects of Ginkgo biloba against carbon tetrachloride induced hepatic injury in rats. Indian J Pharmacol. 2001;33(4):260-6.
-
8.
Lieber CS. Role of oxidative stress and antioxidant therapy in alcoholic and nonalcoholic liver diseases. Adv Pharmacol. 1997;38:601-28. [PubMed ID: 8895826].
-
9.
Cervinkova Z, Drahota Z. Enteral administration of lipid emulsions protects liver cytochrome c oxidase from hepatotoxic action of thioacetamide. Physiol Res. 1998;47(2):151-4. [PubMed ID: 9707000].
-
10.
Sreelatha S, Padma PR, Umadevi M. Protective effects of Coriandrum sativum extracts on carbon tetrachloride-induced hepatotoxicity in rats. Food Chem Toxicol. 2009;47(4):702-8. [PubMed ID: 19146910]. https://doi.org/10.1016/j.fct.2008.12.022.
-
11.
Weber LW, Boll M, Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol. 2003;33(2):105-36. [PubMed ID: 12708612]. https://doi.org/10.1080/713611034.
-
12.
Shui G, Leong LP. Residue from star fruit as valuable source for functional food ingredients and antioxidant nutraceuticals. Food Chem. 2006;97(2):277-84. https://doi.org/10.1016/j.foodchem.2005.03.048.
-
13.
Celik S, Bakırcı I, Şat IG. Physicochemical and organoleptic properties of yogurt with cornelian cherry paste. Int J Food Properties. 2006;9(3):401-8. https://doi.org/10.1080/10942910600596258.
-
14.
Tural S, Koca I. Physico-chemical and antioxidant properties of cornelian cherry fruits (Cornus mas L.) grown in Turkey. Sci Hortic. 2008;116(4):362-6. https://doi.org/10.1016/j.scienta.2008.02.003.
-
15.
Vareed SK, Reddy MK, Schutzki RE, Nair MG. Anthocyanins in Cornus alternifolia, Cornus controversa, Cornus kousa and Cornus florida fruits with health benefits. Life Sci. 2006;78(7):777-84. [PubMed ID: 16139847]. https://doi.org/10.1016/j.lfs.2005.05.094.
-
16.
Serteser A, Kargıoğlu M, Gök V, Bağcı Y, Özcan MM, Arslan D. Antioxidant properties of some plants growing wild in Turkey. Grasas Y Aceites. 2009;60(2). https://doi.org/10.3989/gya.086208.
-
17.
Seeram NP, Schutzki R, Chandra A, Nair MG. Characterization, quantification, and bioactivities of anthocyanins in Cornus species. J Agric Food Chem. 2002;50(9):2519-23. [PubMed ID: 11958615].
-
18.
Koçyiğit M, Özhatay N. Wild plants used as medicinal purpose in Yalova (Northwest Turkey). Turkish J Pharm Sci. 2006;3(2):91-103.
-
19.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265-75. [PubMed ID: 14907713].
-
20.
Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302-10. [PubMed ID: 672633].
-
21.
Fowler WE, Buhle EL, Aebi U. Tubular arrays of the actin-DNase I complex induced by gadolinium. Proc Natl Acad Sci U S A. 1984;81(6):1669-73. [PubMed ID: 6584900].
-
22.
Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70(1):158-69. [PubMed ID: 6066618].
-
23.
Halliwell B, Gutteridge J. Oxygen radicals and the nervous system. Trends Neurosci. 1985;8(1):22-6. https://doi.org/10.1016/0166-2236(85)90010-4.
-
24.
Janero DR. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med. 1990;9(6):515-40. https://doi.org/10.1016/0891-5849(90)90131-2.
-
25.
Marathe GK, Harrison KA, Murphy RC, Prescott SM, Zimmerman GA, McIntyre TM. Bioactive phospholipid oxidation products. Free Radic Biol Med. 2000;28(12):1762-70. [PubMed ID: 10946218].
-
26.
Nagata H, Takekoshi S, Takagi T, Honma T, Watanabe K. Antioxidative action of flavonoids, quercetin and catechin, mediated by the activation of glutathione peroxidase. Tokai J Exp Clin Med. 1999;24(1):1-11. [PubMed ID: 10530620].
-
27.
Reiter RJ, Tan DX, Osuna C, Gitto E. Actions of melatonin in the reduction of oxidative stress. A review. J Biomed Sci. 2000;7(6):444-58. [PubMed ID: 11060493].
-
28.
Baudrimont I, Ahouandjivo R, Creppy EE. Prevention of lipid peroxidation induced by ochratoxin A in Vero cells in culture by several agents. Chem Biol Interact. 1997;104(1):29-40. [PubMed ID: 9158693].
-
29.
Sugiyama T, Nagata J, Yamagishi A, Endoh K, Saito M, Yamada K, et al. Selective protection of curcumin against carbon tetrachloride-induced inactivation of hepatic cytochrome P450 isozymes in rats. Life Sci. 2006;78(19):2188-93. [PubMed ID: 16288784]. https://doi.org/10.1016/j.lfs.2005.09.025.
-
30.
Muriel P, Alba N, Perez-Alvarez VM, Shibayama M, Tsutsumi VK. Kupffer cells inhibition prevents hepatic lipid peroxidation and damage induced by carbon tetrachloride. Comp Biochem Physiol C Toxicol Pharmacol. 2001;130(2):219-26. [PubMed ID: 11574291].
-
31.
Sadasivan S, Latha PG, Sasikumar JM, Rajashekaran S, Shyamal S, Shine VJ. Hepatoprotective studies on Hedyotis corymbosa (L.) Lam. J Ethnopharmacol. 2006;106(2):245-9. [PubMed ID: 16495024]. https://doi.org/10.1016/j.jep.2006.01.002.