Abstract
Background:
Preimplantation genetic diagnosis (PGD) is a diagnostic approach in assisted reproductive technology (ART) to detect and select unaffected embryos to be transferred. Obtaining biopsy samples from embryos (polar body, blastomere, or blastocyst) is a key step in preimplantation genetic testing (PGT), which has many technical issues.Objectives:
This study aimed to evaluate the effect of biopsies from 3-day embryos (blastomere) on the quality of embryos and implantation success in couples who requested sex selection before embryo transfer.Methods:
On the third day after fertilization, 352 high-quality embryos (> six cells on day third with < 10% fragmentation) were collected from 77 women and were tested for sex selection using FISH testing. A laser beam was used to obtain blastomere biopsies by removing a significantly small portion of the zona pellucida. One blastomere was gently biopsied by an aspiration pipette through its hole. After biopsy sampling, the embryo was immediately returned to the embryo scope until transfer. Embryos’ integrity and blastocyst formation were assessed on day 5.Results:
A total of 595 embryos were studied, including 352 embryos that were biopsied on day 3 for gender selection (i.e., the intervention group) and 243 intracytoplasmic sperm injection (ICSI) embryos that did not undergo biopsy (i.e., the control group). Overall, 17.1% of the embryos were abnormal for X or Y chromosomes. Biopsy for PGD was performed 67 - 73 hours after ICSI. Blastomere biopsy taking was significantly associated with blastocyst quality and implantation success.Conclusions:
In this study, after obtaining blastomere biopsies, we investigated the growth process of the embryos according to morphokinetic parameters. Our results showed that blastomere biopsy taking could affect the blastulation of embryos and decrease the success rate of implantation.Keywords
In Vitro Fertilization Blastocyst Blastomere Preimplantation Genetic Diagnosis Implantation Gender Selection
1. Background
In infertility clinics, preimplantation genetic testing (PGT) is offered along with fertilization and in vitro fertilization (IVF) to select superior embryos (1). This method, which is used to diagnose diseases associated with single-gene mutations, sex-linked disorders, and chromosomal abnormalities (2) is very effective in preventing the selective termination of pregnancy in couples who are at risk of transmitting genetic defects (3). This method is not only beneficial for the couples referring to infertility clinics for IVF but also for couples who are naturally fertile but carry pathogenic genes and want to prevent the transmission of inherited genetic defects to their offspring (4). In addition, there is increasing interest in determining the sex of the fetus before implantation, where preimplantation genetic diagnosis (PGD) is applicable as well in both medical and non-medical groups. The choice of medical gender aims to prevent sexually transmitted diseases, but the non-medical choice is only performed to satisfy parents who desire to have children with a preferred gender (5).
The IVF procedure is the same for both infertile and fertile couples, except for the fetal biopsy step, which is performed for genetic testing (6). Embryonic genetic materials for testing can be obtained from three sources (the polar body, blastomere, and blastocyst). In the most common method, sampling is performed in the cleavage stage, in which one or, in some cases, two blastomeres are isolated from a 6- to 8-cell embryo on the third day (7). Biopsy sampling is performed with breaching of the zona pellucida, which can be performed in several ways. Today, the most popular method of elective biopsy of the fetus is to use a laser beam (8). Over the years, significant advances have been made in laboratory standards and the quality of culture media. Good-quality culture media are essential for increasing the quality and evolution of blastocytes (9, 10), causing IVF clinics to consider transferring embryos at the blastocyst stage. There are reports showing that blastocyst implantation rates are higher compared to embryo transfer in the early stages (11). In addition, trophectoderm biopsy has significantly improved and become more popular in infertility centers (12). In vitro fertilization process enables infertility centers to successfully grow the embryo to the blastocyst stage, which is an appropriate stage for biopsy sampling (13). In many studies, trophectoderm biopsies have been successfully performed for genetic testing, after which the embryo has been transferred to the mother’s uterus without any quality changes (14-16).
2. Objectives
In this study, the embryos of two groups of infertile couples (with and without requests for PGD before transferring embryos to the mother’s uterus) were analyzed to assess the effects of biopsy sampling from embryos at the cleavage stage on the success rates of implantation and blastulation.
3. Methods
3.1. Study Design
This retrospective study was conducted on couples referring to the Fertility Clinics of Milad and Shams hospitals in Tabriz, Iran, from August 2020 to July 2021, for sex determination of their embryos, where we also assessed the variations of X and Y chromosomes, their copy numbers, quality of embryos after biopsy sampling, and embryo implantation rate in the couples undergoing assisted reproductive technology (ART) treatments. The PGD process was performed in Dr. Seyed Ali Rahmani Medical Genetics Laboratory.
3.2. Patients
In this study, 154 infertile couples with primary infertility, a mean age of 35 years, were studied. All of the selected couples had normal chromosomal pictures (karyotypes), and no single gene disease was observed in their pedigrees (analyzed for up to three generations). Seventy-seven couples requested PGD testing to determine the sex of their embryos. A total of 595 embryos were formed for these 154 couples, and PGD testing was performed on 352 embryos of the couples who requested sex determination.
3.3. IVF Treatment
Women were administered FSH medications to stimulate the release of multiple eggs during a single ovulation induction cycle. Follicle growth was controlled, and when at least three follicles reached a size of 17 mm, egg maturation and luteinization of the follicles occurred, and egg retrieval was performed 37 hours later. The resulting oocytes were fertilized by intracytoplasmic sperm injection (ICSI) and cultured in human embryo culture media at 37°C with 6% CO2 and 5% O2. The embryos were classified by an embryologist according to morphological criteria into three classes in either the cleavage stage (A, B, and C) or the blastocyst stage (1, 2, and 3) according to Gardner criteria (Table 1) (17).
Classification of Blastocytes Based on Their Quality
| Groups | Quality of Embryos |
|---|---|
| 1 | 5AA/5AB/5BA/5BB |
| 2 | 4AA/4AB/4BA/4BB; 3AA/3AB/3BA/3BB |
| 3 | 2AA/2AB/2BA/2BB; 1AA/1AB/1BA/1BB |
3.4. Biopsy Sampling
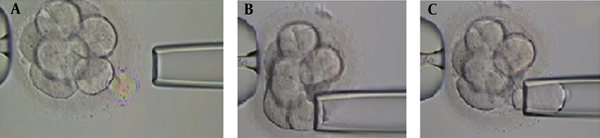
Biopsy sampling from embryos was performed by the same embryologist for all cases. Embryos with 6 - 8 blastomeres with grades A, B, and C were selected for biopsy sampling at the cleavage stage on day third after fertilization. Biopsies were taken after breaching the zona pellucida using the Octax laser (MTG, Germany). The size of the gap is important because creating a large gap damages the embryo. The biopsy was performed with a microinjection microscope (Figure 1). The embryos were placed in a biopsy medium free of calcium and magnesium for 3 to 5 minutes before biopsy sampling to facilitate the process. In order to obtain biopsies using a microinjection microscope, the embryos were held in place by a retaining micropipette. The embryo was then rotated until the blastomere was in the 3 o’clock position for biopsy taking. A hole was made in the zona pellucida using the Octax laser (MTG, Germany), and the blastomere was gently aspirated by a biopsy micropipette and then was completely removed from the embryo (Figure 1). After biopsy sampling, the embryos were washed and grown in the blastocyst growth medium containing serum protein supplements until the day of transfer.
The process of embryo biopsy sampling in the cleavage stage. A, the smaller portion of the zona pellucida was removed by a laser; B, micropipette insertion through the zona; C, blastomere aspiration
3.5. Slide Preparation
Blastomeres were placed in droplets containing a fixative solution (1% tween, 20, and 0.05% hydrochloric acid) at specific locations on a slide; the cytoplasmic membrane was slipped, and the nucleus was then fixed.
3.6. FISH Analysis
The embryos’ sex chromosomes were examined using a FISH Probe (Cat NO. LPH002). At first, the slide was washed in 2X sodium saline citrate buffer for two minutes and then was placed in 70%, 85%, and 100% ethanol, respectively, each for one minute. Then ten μL of the probe was placed on the slide, which was then covered by a cover slip. The slides were placed at 37°C for five min, followed by denaturation at 75°C for two minutes. Then the slides were incubated at 37°C for hybridization, and after 24 hours, the cover slip was removed, and the slides were washed for two minutes in a 72°C sodium saline buffer and then one minute in a sodium citrate buffer containing 0.05% tween 20. Eventually, ten μL of Depi was placed on the slide, which was covered with a cover slip afterward. The prepared slide was studied by a 410Motic BA fluorescent microscope applying Dapi, FITC, and TEXAS RED filters.
3.7. Embryo Transfer
The patients were divided into two groups; no PGD testing (n = 77 couples who did not request this test) and PGD testing for sex determination. In both groups, two embryos were transferred to the mother’s uterus in the blastocyst stage. In both groups, two embryos were transferred to the mother's uterus. For the parents requesting PGD testing, embryos with the desired sex were selected for transfer.
3.8. Statistical Analysis
Categorical data were presented as frequencies and percentages. The comparison of frequency between the groups was performed using the chi-square test. Also, relationships between variables were measured by the Spearman correlation coefficient and gamma coefficient. Analyses were conducted in statistical package for the social sciences (SPSS) software (SPSS version 26.0, SPSS Inc., Chicago, IL, USA).
4. Results
4.1. Clinical Characteristics of Couples
Table 2 shows the clinical characteristics of the studied couples (154 couples); 42.1% of women were under 35 years of age. Also, 35.1% of the women who requested PGD and 40.3% of the women who did not request PGD were under 35 years of age. The age of men ranged from 30 to 55 years, 42% of whom were younger than 38 years old. Overall, IVF failed in 37 (48.1%) of the couples who requested PGD and 44 (57.1%) of the couples who did not request this test. All the couples were treated with ICSI/IVF with their own eggs and sperm.
Characteristics of the Couples Included in the Study, Including Those Who Had (Group 1) or Had Not (Group 2) Requests for Preimplantation Genetic Diagnosis a
| Characteristics | Group 1 | Group 2 |
|---|---|---|
| Maternal age in years, median (range) | 35 (30 - 46) | 35 (30 - 50) |
| 30 - 35 | 30 (39) | 27 (35.1) |
| 35 - 40 | 34 (44.2) | 31 (40.3) |
| 40 - 45 | 9 (11.7) | 13 (16.9) |
| 45 - 50 | 4 (5.2) | 5 (6.5) |
| 50 - 55 | 0 | 1 (1.3) |
| Number of women (%) ≤ 35 | 30 (39) | 36 (46.8) |
| Number of women (%) > 35 | 47 (61) | 41 (53.2) |
| Number of embryos | 243 | 352 |
| Number of embryos with quality A (day 3) | 155 (63.8) | 201 (57.1) |
| Number of embryos with quality B (day 3) | 50 (20.6) | 87 (24.7) |
| Number of embryos with quality C (day 3) | 38 (15.6) | 64 (18.2) |
| Number of arrested embryos | 30 (5) | 69 (11.6) |
| Number of embryos (group 1 quality in Table 1) | 161 (27.1) | 100 (16.8) |
| Number of embryos (group 2 quality in Table 1) | 37 (6.4) | 89 (14.8) |
| Number of embryos (group 3 quality in Table 1) | 15 (2.5) | 94 (15.8) |
| Male factors | 35 (47.7) | 29 (37.7) |
| Primary infertility | 77 (100) | 77 (100) |
| Previous IVF failure | 37 (48.1) | 44 (57.1) |
| Number of total ICSI/IVF cycles | 77 (100) | 77 (100) |
4.2. Genetic Analysis Findings
Overall, 40.8% of 352 studied embryos were XX, and 33% of them were XY; 15.4% of them were aneuploid, and 1.7% were euploid. The highest rate of aneuploidy was related to XO, which included 7.4% of all abnormal embryos; 4% were XXY, 2.3% were XYY, and 1.7% were XXX. In 9.1% of the embryos assessed, the nucleus was lost during the fixation process or detached from the slide during the washing process.
4.3. Relationship Between Male Infertility Factors and the Quality of Embryos
Among the couples requesting or not requesting PGD, 29 (37.7%) and 35 (47.7%) of men had fertility problems, respectively, characterized by low (mild to severe) sperm counts and abnormal sperm motility with normal karyotype. Overall, 41.2% of embryos with high quality on the day third after fertilization belonged to this group of men. No significant correlation was found between the quality of embryos and male fertility status (P = 0.34).
4.4. Relationship Between Embryos’ Quality and Implantation
Transferring to the uterus was performed for 49 (31.8%), 4 (2.6%), 10 (6.5%), and 15 (9.7%) women who had embryos with grades 1, 2, 3, and all grade quality, respectively, and after two weeks, beta-HCG testing rendered positive results for 15 (39.5%), 2 (3.1%), and 3 (7.9%) of the women who quality grade 1, 2, and 3 embryos, respectively. No significant relationship was observed between the rate of embryo implantation and the quality of transferred embryos (P = 0.27).
4.5. The Effect of Blastomere Biopsy Sampling on Implantation
The embryos were transferred to women’s uteruses, and implantation was controlled by beta-HCG measurement after two weeks. Implantation success was compared between the two groups of women (i.e., with or without PGD testing), where 29 (37.7%) of those without PGD testing and 18 (23.4%) of women with PGD testing were pregnant. The results showed a significant relationship between blastomere biopsy sampling and successful implantation (P = 0.04).
4.6. Impact of Blastomere Biopsy Sampling on Embryos’ Growth
Out of embryos undergoing blastomere biopsy sampling on day third, 201 (57.1%), 87 (24.7%), and 64 (18.2%) embryos with grade A, B, and C quality, respectively, continued development and growth, and the respective values were 155 (63.8%), 50 (20.6%), and 38 (15.6%) for embryos that did not undergo biopsy sampling. The comparison of the quality of embryos in the blastocyst stage (Table 2) revealed a significant difference in embryos’ development between the two groups (i.e., biopsied and non-biopsied) (P = 0.01). In other words, a significant relationship was observed between blastomere biopsy sampling and the quality and development of embryos.
5. Discussion
The PGD technique is recommended for older couples (< 35 years old), those experiencing repeated implantation failure (RIF), recurrent pregnancy loss (RPL), and carriers of single-gene and chromosomal diseases. This method is used to increase the rate of healthy pregnancies and healthy live births through IVF treatment (18). In this study, the effect of blastomere biopsy sampling was assessed on the success rate of implantation and on embryo quality. In our study, 62.7% of implantation efforts were successful, and 61.8% of high-quality embryos in the blastocyst stage were related to the embryos that did not undergo biopsy sampling. The results of our study were consistent with the findings of Bar-El et al., who showed that blastomere biopsy sampling delayed the compaction and blastulation of embryos and reduced the success rate of implantation (19). The use of these technologies for the genetic studying of embryos increases the rate of healthy live births by detecting chromosomal abnormalities in embryos before transfer (20). On the other hand, no instructions have been provided for the optimal time of biopsy. In most laboratories, biopsy sampling is performed at the cleavage stage to allow sufficient time for genetic analysis (21). In a study, Ashiru et al. compared the effects of biopsy sampling at the cleavage or blastocyst stage and showed a significant difference in the success rate of implantation between embryos undergoing biopsy in the two different stages mentioned (22). Also, our results showed that the blastulation rate was significantly reduced in embryos undergoing biopsy at the cleavage stage, which was consistent with the report of Vega et al., who noted that biopsy sampling at the cleavage stage reduced the overall proportion of the embryos growing to the blastocyst stage by 25 percent (23). Shi et al. showed that chromosome screening had beneficial effects in pregnancies with advanced maternal age. Moreover, biopsy sampling at the blastocyst stage had a better outcome compared to the polar body and cleavage stages (24). Kalma et al. showed that blastomere biopsy could be less harmful to embryos’ development if it is carried out during a critical period of embryonic growth, i.e., during the 8-cell stage. They also demonstrated the added value of time-lapse microscopy for determining the optimal timing for blastomere biopsy taking (21). In a study conducted by Aghajanova et al., similar to our study, they did not find a significant relationship between sperm quality and embryos’ morphological features (25). However, Piccolomini et al. argued that sperm quality affected embryos’ growth and development, evidenced by a decrease in the blastulation rate in embryos from low-quality sperms (26). The results of the recent study contradicted our observation, which may be related to the fact that we used the sperm intracytoplasmic injection method, which can reduce the negative impacts of low-quality sperm on treatment outcomes. This study is the first report on the Azerbaijani population of Iran. Overall, the results of our study were comparable to previous global reports.
5.1. Conclusions
Embryo biopsy sampling at the cleavage stage affected the development and blasting processes of embryos and reduced the success rate of implantation. On the other hand, the PGD technique not only allows for gender detection of embryos but also identifies sex chromosome abnormalities, so the transfer of abnormal embryos to the uterus can be prevented, highlighting the importance of this technique. It is necessary to increase the sample size and collect more data from several infertility clinics to find any correlation between embryo biopsy sampling and the studied parameters. The advantages and disadvantages of embryo manipulation technologies should also be further investigated, and the studied parameters are suggested to be assessed for trophyctoderm biopsy sampling in the blastocyte stage as well.
Acknowledgements
References
-
1.
Harper JC, Sengupta SB. Preimplantation genetic diagnosis: state of the art 2011. Hum Genet. 2012;131(2):175-86. [PubMed ID: 21748341]. https://doi.org/10.1007/s00439-011-1056-z.
-
2.
Stern HJ. Preimplantation Genetic Diagnosis: Prenatal Testing for Embryos Finally Achieving Its Potential. J Clin Med. 2014;3(1):280-309. [PubMed ID: 26237262]. [PubMed Central ID: PMC4449675]. https://doi.org/10.3390/jcm3010280.
-
3.
Harper JC, Boelaert K, Geraedts J, Harton G, Kearns WG, Moutou C, et al. ESHRE PGD Consortium data collection V: cycles from January to December 2002 with pregnancy follow-up to October 2003. Hum Reprod. 2006;21(1):3-21. [PubMed ID: 16172150]. https://doi.org/10.1093/humrep/dei292.
-
4.
Harper JC, Coonen E, De Rycke M, Harton G, Moutou C, Pehlivan T, et al. ESHRE PGD Consortium data collection X: cycles from January to December 2007 with pregnancy follow-up to October 2008. Hum Reprod. 2010;25(11):2685-707. [PubMed ID: 20813804]. https://doi.org/10.1093/humrep/deq228.
-
5.
Panahi S, Fahami F. The results of pregnancies after gender selection by pre implantation genetic diagnosis and its relation with couple's age. Iran J Nurs Midwifery Res. 2015;20(6):670-5. [PubMed ID: 26793251]. [PubMed Central ID: PMC4700685]. https://doi.org/10.4103/1735-9066.170012.
-
6.
Harton GL, Magli MC, Lundin K, Montag M, Lemmen J, Harper JC, et al. ESHRE PGD Consortium/Embryology Special Interest Group--best practice guidelines for polar body and embryo biopsy for preimplantation genetic diagnosis/screening (PGD/PGS). Hum Reprod. 2011;26(1):41-6. [PubMed ID: 20966459]. https://doi.org/10.1093/humrep/deq265.
-
7.
Fernandez SF, Toro E, Colomar A, Lopez-Teijon M, Velilla E. A 24-chromosome FISH technique in preimplantation genetic diagnosis: validation of the method. Syst Biol Reprod Med. 2015;61(3):171-7. [PubMed ID: 25582218]. https://doi.org/10.3109/19396368.2014.1002869.
-
8.
Montazeri F, Foroughmand AM, Kalantar SM, Aflatoonian A, Khalilli MA. Tips and Tricks in Fluorescence In-situ Hybridization (FISH)- based Preimplantation Genetic Diagnosis /Screening (PGD/PGS). Int J Med Lab. 2018;5(2):84-98.
-
9.
Machtinger R, Racowsky C. Morphological systems of human embryo assessment and clinical evidence. Reprod Biomed Online. 2013;26(3):210-21. [PubMed ID: 23352813]. https://doi.org/10.1016/j.rbmo.2012.10.021.
-
10.
Stensen MH, Tanbo T, Storeng R, Byholm T, Fedorcsak P. Routine morphological scoring systems in assisted reproduction treatment fail to reflect age-related impairment of oocyte and embryo quality. Reprod Biomed Online. 2010;21(1):118-25. [PubMed ID: 20452822]. https://doi.org/10.1016/j.rbmo.2010.03.018.
-
11.
Fesahat F, Montazeri F, Sheikhha MH, Saeedi H, Dehghani Firouzabadi R, Kalantar SM. Frequency of chromosomal aneuploidy in high quality embryos from young couples using preimplantation genetic screening. Int J Reprod Biomed. 2017;15(5):297-304. [PubMed ID: 28744525]. [PubMed Central ID: PMC5510583].
-
12.
Rubio C, Rodrigo L, Mir P, Mateu E, Peinado V, Milan M, et al. Use of array comparative genomic hybridization (array-CGH) for embryo assessment: clinical results. Fertil Steril. 2013;99(4):1044-8. [PubMed ID: 23394777]. https://doi.org/10.1016/j.fertnstert.2013.01.094.
-
13.
de Boer KA, Catt JW, Jansen RP, Leigh D, McArthur S. Moving to blastocyst biopsy for preimplantation genetic diagnosis and single embryo transfer at Sydney IVF. Fertil Steril. 2004;82(2):295-8. [PubMed ID: 15302271]. https://doi.org/10.1016/j.fertnstert.2003.11.064.
-
14.
Kokkali G, Vrettou C, Traeger-Synodinos J, Jones GM, Cram DS, Stavrou D, et al. Birth of a healthy infant following trophectoderm biopsy from blastocysts for PGD of beta-thalassaemia major. Hum Reprod. 2005;20(7):1855-9. [PubMed ID: 15878929]. https://doi.org/10.1093/humrep/deh893.
-
15.
McArthur SJ, Leigh D, Marshall JT, de Boer KA, Jansen RP. Pregnancies and live births after trophectoderm biopsy and preimplantation genetic testing of human blastocysts. Fertil Steril. 2005;84(6):1628-36. [PubMed ID: 16359956]. https://doi.org/10.1016/j.fertnstert.2005.05.063.
-
16.
Lee A, Kiessling AA. Early human embryos are naturally aneuploid-can that be corrected? J Assist Reprod Genet. 2017;34(1):15-21. [PubMed ID: 27900612]. [PubMed Central ID: PMC5330987]. https://doi.org/10.1007/s10815-016-0845-7.
-
17.
Shi D, Xu J, Zhang M, Niu W, Shi H, Yao G, et al. Association between the quality of inner cell mass and first trimester miscarriage after single blastocyst transfer. Reprod Biol Endocrinol. 2020;18(1):43. [PubMed ID: 32398002]. [PubMed Central ID: PMC7216576]. https://doi.org/10.1186/s12958-020-00595-y.
-
18.
Cardenas-Nieto D, Forero-Castro M, Moreno-Ortiz H, Lucena-Quevedo E, Cuzzi J, Esteban-Perez C. Analysis of a Preimplantation Genetic Test for Aneuploidies in Embryos from Colombian Couples: A Report of Cases. J Reprod Infertil. 2020;21(1):17-33. [PubMed ID: 32175262]. [PubMed Central ID: PMC7048689].
-
19.
Bar-El L, Kalma Y, Malcov M, Schwartz T, Raviv S, Cohen T, et al. Blastomere biopsy for PGD delays embryo compaction and blastulation: a time-lapse microscopic analysis. J Assist Reprod Genet. 2016;33(11):1449-57. [PubMed ID: 27696105]. [PubMed Central ID: PMC5125156]. https://doi.org/10.1007/s10815-016-0813-2.
-
20.
Friedenthal J, Maxwell SM, Munne S, Kramer Y, McCulloh DH, McCaffrey C, et al. Next generation sequencing for preimplantation genetic screening improves pregnancy outcomes compared with array comparative genomic hybridization in single thawed euploid embryo transfer cycles. Fertil Steril. 2018;109(4):627-32. [PubMed ID: 29605407]. https://doi.org/10.1016/j.fertnstert.2017.12.017.
-
21.
Kalma Y, Bar-El L, Asaf-Tisser S, Malcov M, Reches A, Hasson J, et al. Optimal timing for blastomere biopsy of 8-cell embryos for preimplantation genetic diagnosis. Hum Reprod. 2018;33(1):32-8. [PubMed ID: 29165686]. https://doi.org/10.1093/humrep/dex343.
-
22.
Ashiru OA, Ogbeche RO, Oladimeji MO, Iloabachie EC, Umana AE, Osumah JG. Blastocyst and cleavage stage embryo biopsy for preimplantation genetic testing of the sickle cell gene in carrier couples: the experience of an IVF clinic in a developing country: a retrospective study. Glob Reprod Health. 2018;3(3):e17. https://doi.org/10.1097/grh.0000000000000017.
-
23.
Vega M, Breborowicz A, Sauerbrun M, Stein D, McGovern P, Keltz M. Day 3 Biopsy and Blastulation Rates. J Reprod Med. 2016;61(7-8):336-40. [PubMed ID: 30408378].
-
24.
Shi WH, Jiang ZR, Zhou ZY, Ye MJ, Qin NX, Huang HF, et al. Different Strategies of Preimplantation Genetic Testing for Aneuploidies in Women of Advanced Maternal Age: A Systematic Review and Meta-Analysis. J Clin Med. 2021;10(17):3895. [PubMed ID: 34501345]. [PubMed Central ID: PMC8432243]. https://doi.org/10.3390/jcm10173895.
-
25.
Aghajanova L, Kao CN, Cedars M, Tran N. Assessing the impact of semen quality on embryo development in an egg donation model. F S Rep. 2021;2(1):22-9. [PubMed ID: 34223269]. [PubMed Central ID: PMC8244319]. https://doi.org/10.1016/j.xfre.2020.10.012.
-
26.
Piccolomini MM, Bonetti TC, Motta E, Serafini PC, Alegretti JR. How general semen quality influences the blastocyst formation rate: Analysis of 4205 IVF cycles. JBRA Assist Reprod. 2018;22(2):89-94. [PubMed ID: 29672007]. [PubMed Central ID: PMC5982551]. https://doi.org/10.5935/1518-0557.20180022.