Abstract
Background:
Prenatal rat embryo exposure to retinoid induces some malformations in various organs, the most active and teratogenic metablolite is all-trans-retinoic acid (atRA). The teratogenic effects of some drugs can be prevented by the application of antioxidant drugs and stimulation of the maternal immune system. Also, quercetin, a naturally occurring flavonoid has excellent antioxidant properties. Therefore, in this study, the prophylactic effect of quercetin on teratogenic effects of atRA was evaluated.Materials and Methods:
In this experimental study, 40 pregnant rats were divided into 7 groups. Control group received normal saline and test groups received dimethylsulfoxide (DMSO), quercetin (75 mg/kg), quercetin (200 mg/kg), atRA (25 mg/kg), atRA (25 mg/kg) plus quercetin (75 mg/kg) and atRA (25 mg/kg) plus quercetin (200 mg/kg), intraperitoneally at 8-10th days of gestation. Fetuses were collected at 20th day of gestation and after determination of weight and length; they were stained by Alizarin red-Alcian blue method.Results:
Cleft palate, exencephaly and spina bifida incidence were 30.76%, 61.53% and 30.76% range in group which received only atRA. Cleft palate, exencephaly and spina bifida incidence were 11.11%, 16.66% and 5.55% in group which received atRA plus quercetin (75 mg/kg). However, cleft palate, exencephaly and spina bifida incidence were 10.52%, 10.52% and 0% in group which received atRA plus quercetin (200 mg/kg). The means of weight and length of fetuses from rat that received atRA plus quercetin (75 mg/kg) were significantly greater than those received only atRA.Conclusion:
It is concluded that quercetin decreased teratogenicity induced by atRA, but this subject needs more detailed evaluation.Keywords
Retinoic acid Quercetin Pregnancy Cleft palate Teratogenicity Fetus Rat
Introduction
All-trans-retinoic acid (atRA), a bioactive vitamin A metabolite, is a signaling molecule indispensable for the formation of many organs, including eyes, heart, and kidneys [1]. atRA is an important physiological regulator of cellular differentiation, proliferation, apoptosis, reproduction and embryonic development in many species [2]. Retinoic action is mediated by specific nuclear retinoic acid receptors and retinoid receptors belonging to the steroid/thyroid super-family of transcription factors [3]. Inadequate levels of retinoids (excess or deficiency) may result in a set of defects denoted retinoic acid embryopathy which may provoke defects in the development of the neural crista [4], in addition to limb malformation [5] and other skeletal malformations [6].
atRA affects by serving as an activating ligand of nuclear atRA receptors (RAR α, β, and γ) and peroxisome proliferator-activated receptors (PPAR β/δ), which form heterodimers with retinoid X receptors [7]. The concentration of atRA during embryonic development is tightly controlled in a spatial and temporal manner, and in adult tissues, it is maintained within a very narrow range that is specific for each given tissue. If the control mechanisms fail and the concentration of atRA exceed or fall below the optimal range, tissues and cells undergo pathophysiological changes that in most severe cases can lead to disease [8].
Embryopathy due to RA is being intensely investigated in view of the teratogenic potential of retinols and of the crucial role played by their receptors in embryo development. The RA dose of 70 mg/kg was administered in mice on the eighth gestational day which caused neural tube defect (NTD), omphalocele, gastroschisis, limb defects, imperforated anus and tail agenesis/alteration. atRA increases the production of reactive oxygen species and oxidative stress [9].
On the other hand, a group of supplements that are good candidates for antioxidant therapy are flavonoids. Flavonoids are a class of polyphenolic compounds available in the fruits and vegetables. Epidemiological studies showed that the risk of cardiovascular disease in subjects, who had a high intake of flavonoids, has been reduced [10]. Quercetin (3, 30, 40, 5, 7-pentahydroxy-flavone) is a flavonoid commonly found in frequently consumed foods, including apples, berries, onion, tea, nuts, seed and vegetables that represent an integral part of the human diet. Quercetin is one of the most abundant representing the 60%-75% of the average polyphenol ingestion [11]. Quercetin has been reported to have biological, pharmacological, and medicinal activities [12] that are believed to arise from its antioxidant properties [13].
Quercetin was reported to have many beneficial effects on human health, including cardiovascular protection, anticancer activity, antiulcer activity, anti-allergic activity, cataract prevention, anti-inflammatory and antiviral activity [14]. Quercetin could prevent oxidant injury and cell death by several mechanisms, such as scavengering oxygen radicals, protecting against lipid peroxidation and chelating metal ions [15]. Quercetin directly scavenges the superoxide anion [16] and inhibits several superoxide-generating enzymes such as xanthine oxidase (XO) [17] or the neutrophil membrane NADPH oxidase complex [18]. As mentioned above atRA increases oxidative stress and quercetin acts as antioxidant; so, in present study, the preventive effect of quercetin on atRA-induced neural tube and skeletal malformations in rats was evaluated.
Results
No maternal deaths were observed throughout the course of this study. Likewise, the dose of atRA used in this investigation was well tolerated by the dams, as evidenced by no differences in food and water consumption.
Fourty two fetuses were obtained from seven rats of control group. There were not observed macroscopic anomalies in the control animals. In the control group palatal closures of fetuses were normal (Fig. 1, 2-A) at gestational day 20 (i.e., palatal shelves had grown vertically on the sides of the tongue, then horizontally to meet and fuse).
atRA induced cleft palate, exencephaly and spina bifida 30.76%, 61.53% and 30.76% incidence respectively. Quercetin with dose of 75 mg/kg reduced incidence of atRA-induced cleft palate, exencephaly and spina bifida to 11.11%, 16.66% and 5.55% respectively (Fig. 1, 2, 3). Quercetin 200 mg/kg reduced incidence of atRA-induced cleft palate, exencephaly and spina bifida to 10.52%, 10.52% and 0% respectively.
Percentages of absorbed fetuses were 66.66, 47.05 and 45.71 in groups 2, 6 and 7, respectively, so quercetin decreased the resorption rate.
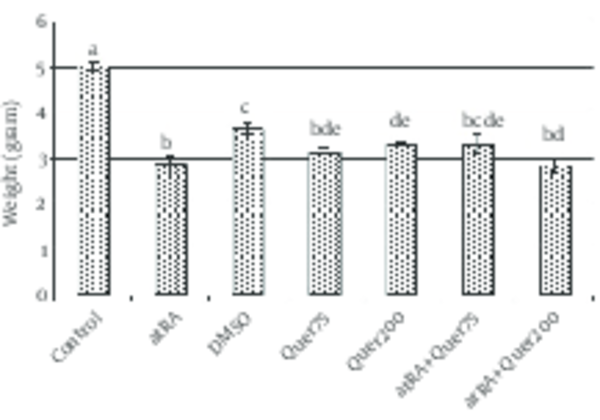
The mean of weight of animals' fetuses that received atRA in 8-10th days was significantly (p<0.0001) decreased in comparison with normal saline group. The mean of length of animals' fetuses that received atRA plus quercetin (75 mg/kg) in 8-10th days was significantly (p<0.0001) greater than the group received only atRA.
The mean weight and length in the group that received quercetin (200 mg/kg) did not differ significantly with atRA group.
The mean weight (p<0.0001) and length (p<0.0001) were significantly decreased in the group which received quercetin in comparison to the control group (Fig. 4, 5).
Incidence of anomalies in fetuses of three study groups
| Anomaly | Incidence (%) | ||
|---|---|---|---|
| Group 2 | Group 6 | Group 7 | |
| 30.76 | 11.11 | 10.52 | |
| 61.53 | 16.66 | 10.52 | |
| 30.76 | 5.55 | 0 | |
Group 2 received all-trans-retinoic acid (atRA) (25 mg/kg); Group 6 received atRA+quercetin (75 mg/kg); Group 7 received atRA+quercetin (200 mg/kg)
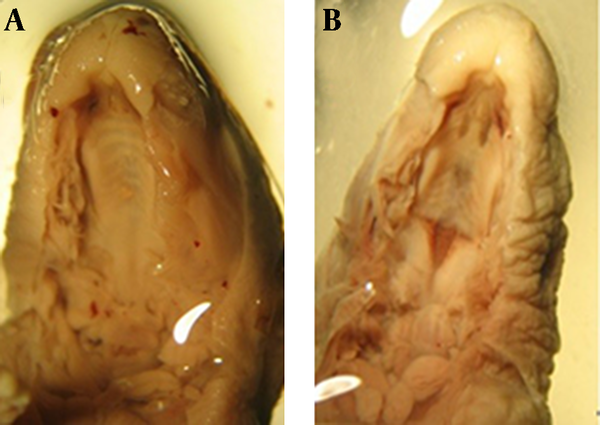
A, B: Razor blade sections of rat fetuses of GD 20. A: Control skeleton. Note the cleft palate due to palatal shelf hypoplasia (B) in the treated case (25 mg/kg of atRA, treated on GD 8-10)
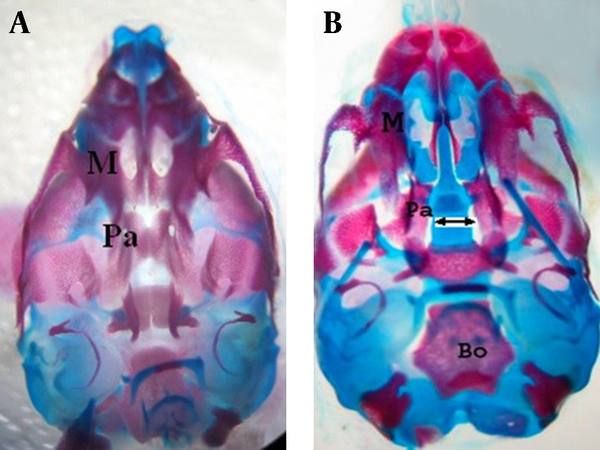
Ventral view of skull of rat fetuses of GD 20, stained with alizarin red S-alcian blue. A) Normal palatine bone B) Cleft palate induced by atRA (arrow)
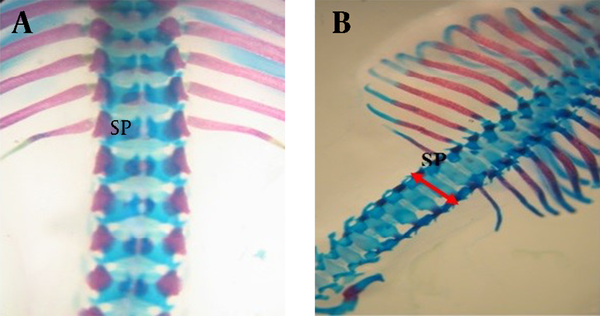
Dorsal view of vertebral column of gestation 20th day fetal rat. A) Normal B) Spina bifida (arrow) induced by atRA which stained with Alizarin red- Alcian blue
Length (mean±SEM) of fetuses in normal saline group and test groups
Weight (mean±SEM) of fetuses in normal saline group and test groups
Discussion
We demonstrated atRA (at dose 25 mg/kg, i.p.) decreased weight and length and produced cleft palate (30.76% present of fetuses), exencephaly (61.53% present of fetuses) and spina bifida (30.76% present of fetuses).
The results presented here show that quercetin administration during the gestational period has a partial protective effect on atRA-induced teratogenesis (decreasing the frequencies of exencephaly, cleft palate, spina bifida). It is well established that atRA is an important physiological regulator of embryonic development; it regulates many processes in organogenesis such as development of important organs and systems including the heart, the cardiovascular system, the hindbrain, and the foregut, among others [6]. However, both its deficiency and excess can result in abnormal embryonic development. When atRA is administered in large doses during this critical period GD 8-10, it causes embryonic malformations in a dose-dependent manner [20]. Inappropriate gene expression has been proposed as a mechanistic basis for atRA teratogenicity. Morphological changes visible after atRA treatment of embryos could be explained by alterations in the spatial and temporal patterns of expression of genes controlling differentiation, proliferation, apoptosis, and morphogenesis in embryonic organization and in initial axial patterning [21].
Kistler reported that retinoic acid (120 mg/kg) was orally administered to pregnant females was highly embryolethal when administered on days 9 and 10 of gestation (96.2 and 100% resorptions). The earliest teratogenic effect of retinoic acid was noted on the 9th day of gestation. Severe multiple defects were produced by retinoic acid administration on days 9 and 11 of gestation, but more specific malformations involving the axial skeleton, the fore- and hind limbs, and cleft palate resulted from treatment on days 12-18 of gestation [22].
Tom et al. reported after maternal treatment of mouse with 5 mg/kg retinoic acid on 8.5 days of gestation, 53% of SELH/Bc mouse embryos had exencephaly, compared with 22% in ICR/Bc and 14% in normal strain (SWV/Bc) [23]. Dorko et al. reported that the application of atRA on 7-9th day of gestation influenced the weight of new-born rats [24].
A wide spectrum of congenital abnormalities, including exophthalmos, microphthalmia and anophthalmia, maxillo-mandibular dysostosis, micrognathia of both maxilla and mandible, cleft palate, subdevelopment of ear lobe, preauricular tags and macroglossia, were observed in the offspring of retinoic acid treated animals. The abnormalities were both time and dosage dependent and characteristic of Treacher-Collins syndrome when retinoic-acid was administered on the 11.5 days of gestational age. In contrast, when retinoic acid was administered were on gestational days 10-12, the defects were similar to those seen in the first and second pharyngeal arch syndrome, as well as in the oculo-auriculo-vertebral spectrum [25].
atRA plays important role in the control of cell differentiation and morphogenesis during prenatal development [26]. However atRA, used in the treatment of dermatological disorders, has been implicated in the production of congenital anomalies in infants born to mothers taking the drugs during the first trimester [27]. In mice, administration of RA leads to small embryo forebrain and hindbrain, exencephaly, little or no flexure of the brain, and optic vesicle aplasia [28]. Similar studies have shown that atRA given after the peri-implantational period induces abdominal wall and neural defects [29]. Our findings are consistent with previous research.
In one study, observed administration time-dependent changes in the teratological effects. Maternal administration of atRA in group II rats was associated with congenital malformations including microcephaly, exencephaly, hydrocephaly, gastrochisis, omphalocele, exophthalmus, mandibular hypoplasia, facial dysmorphia, limb reduction defects and reduction of the crown-rump length. Colakoğlu and Kükner conclude that atRA is more teratogenic before neurulation. The atRA administration during this period leads to severe developmental retardation and malformed stillborns. atRA is less teratogenic after neurulation, and causes only a few omphalocele [30].
Also, we observed protective effect of quercetin on atRA teratogenicity. This effect reported by some researchers. For example; Prater et al. reported that low-dose quercetin (66 mg/kg supplemented in rodent chow throughout gestation; approximately 70% of human dose), high-dose quercetin (333 mg/kg supplemented in rodent chow throughout gestation; approximately 3.5x daily human dose), impairs placental oxidative stress and fetal skeletal malformation induced by methylnitrosourea [31].
Song et al. reported that quercetin has protective effects on the spinal cord by the potential mechanism of inhibiting the activation of p38MAPK/iNOS signaling pathway and thus regulating secondary oxidative stress [32]. Gupta et al. reported that quercetin (10, 30 and 100 mg/kg for 5 consecutive days) ameliorates the diethylnitrosamine induced hepatotoxicity in rats and can be a candidate for a good chemoprotectant [33].
In one study, quercetin was administered at a dose of 10 mg/kg/day, i.p. for 14 days, which results call into question the ability of therapy with the antioxidant quercetin to reverse diabetic oxidative stress in an overall sense [34]. In another study, quercetin treatment prevents renal tubular damage and increased oxidative stress induced by chronic cadmium administration, most probably throughout its antioxidant properties [35].
Also, quercetin reduced abnormal development of mouse embryos produced by hydroxyurea [36]. Liang et al. demonstrated that quercetin (66 mg/kg supplemented diet) significantly improves high fatty saturated induced fetal skeletal maldevelopment, perhaps in part due to antioxidant effects of quercetin in placenta. This speculation is supported by previous reports that demonstrate quercetin prevention of oxidant injury and cell death by ROS scavenging and protection against lipid peroxidation [37].
Abdelmoaty et al. reported that quercetin could prevent hyperglycemia induced by stereptozotosin in rats [38]. In another study, quercetin with dose 50 mg/kg orally was most effective in preventing arsenic poisoning by reducing oxidative stress [39].
In conclusion, the present study showed the effects of quercetin for the first time on teratogenicty induced atRA in rat fetuses. The present results indicate that exposure 25 mg/kg of atRA in 8-10th days of gestation of rat decreases weight and length of embryos and influences on skeletal system. The protective effect of quercetin in atRA-induced teratogenesis in rat may, at least in part, be due to its antioxidant activity, which we believe deserves further investigation.
Acknowledgements
References
-
1.
Duester G. Retinoic acid synthesis and signaling during early organogenesis. Cell. 2008;134(6):921-31. [PubMed ID: 18805086]. https://doi.org/10.1016/j.cell.2008.09.002.
-
2.
Maden M. Retinoids and spinal cord development. J Neurobiol. 2006;66(7):726-38. [PubMed ID: 16688770]. https://doi.org/10.1002/neu.20248.
-
3.
Ross SA, McCaffery PJ, Drager UC, De Luca LM. Retinoids in embryonal development. Physiol Rev. 2000;80(3):1021-54. [PubMed ID: 10893430].
-
4.
Kam RK, Deng Y, Chen Y, Zhao H. Retinoic acid synthesis and functions in early embryonic development. Cell Biosci. 2012;2(1):11. [PubMed ID: 22439772]. https://doi.org/10.1186/2045-3701-2-11.
-
5.
Ali-Khan SE, Hales BF. Novel retinoid targets in the mouse limb during organogenesis. Toxicol Sci. 2006;94(1):139-52. [PubMed ID: 16772331]. https://doi.org/10.1093/toxsci/kfl037.
-
6.
Zile MH. Vitamin A and embryonic development: An overview. J Nutr. 1998;128(2):455-8.
-
7.
Mark M, Ghyselinck NB, Chambon P. Function of retinoid nuclear receptors: lessons from genetic and pharmacological dissections of the retinoic acid signaling pathway during mouse embryogenesis. Annu Rev Pharmacol Toxicol. 2006;46:451-80. [PubMed ID: 16402912]. https://doi.org/10.1146/annurev.pharmtox.46.120604.141156.
-
8.
Rial E, Gonzalez-Barroso M, Fleury C, Iturrizaga S, Sanchis D, Jimenez-Jimenez J, et al. Retinoids activate proton transport by the uncoupling proteins UCP1 and UCP2. EMBO J. 1999;18(21):5827-33. [PubMed ID: 10545094]. https://doi.org/10.1093/emboj/18.21.5827.
-
9.
Notario B, Zamora M, Vinas O, Mampel T. All-trans-retinoic acid binds to and inhibits adenine nucleotide translocase and induces mitochondrial permeability transition. Mol Pharmacol. 2003;63(1):224-31. [PubMed ID: 12488555].
-
10.
Huxley RR, Neil HA. The relation between dietary flavonol intake and coronary heart disease mortality: a meta-analysis of prospective cohort studies. Eur J Clin Nutr. 2003;57(8):904-8. [PubMed ID: 12879084]. https://doi.org/10.1038/sj.ejcn.1601624.
-
11.
Goldberg DM, Hahn SE, Parkes JG. Beyond alcohol: beverage consumption and cardiovascular mortality. Clin Chim Acta. 1995;237(1-2):155-87. [PubMed ID: 7664473].
-
12.
Perez Vizcaino F, Duarte J, Jimenez R. Antihypertensive effects of the flavonoid quercetin. Pharmacol Rep. 2009;61(1):67-75.
-
13.
Boots AW, Haenen GR, Bast A. Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol. 2008;585(2-3):325-37. [PubMed ID: 18417116]. https://doi.org/10.1016/j.ejphar.2008.03.008.
-
14.
Maciel RM, Costa MM, Martins DB, Franca RT, Schmatz R, Graca DL, et al. Antioxidant and anti-inflammatory effects of quercetin in functional and morphological alterations in streptozotocin-induced diabetic rats. Res Vet Sci. 2013;95(2):389-97. [PubMed ID: 23706762]. https://doi.org/10.1016/j.rvsc.2013.04.028.
-
15.
Sanhueza J, Valdes J, Campos R, Garrido A, Valenzuela A. Changes in the xanthine dehydrogenase/xanthine oxidase ratio in the rat kidney subjected to ischemia-reperfusion stress: preventive effect of some flavonoids. Res Commun Chem Pathol Pharmacol. 1992;78(2):211-8. [PubMed ID: 1475527].
-
16.
Robak J, Gryglewski RJ. Flavonoids are scavengers of superoxide anions. Biochem Pharmacol. 1988;37(5):837-41. https://doi.org/10.1016/0006-2952(88)90169-4.
-
17.
Chang WS, Lee YJ, Lu FJ, Chiang HC. Inhibitory effects of flavonoids on xanthine oxidase. Anticancer Res. 1993;13(6A):2165-70. [PubMed ID: 8297130].
-
18.
Tauber AI, Fay JR, Marletta MA. Flavonoid inhibition of the human neutrophil NADPH-oxidase. Biochem Pharmacol. 1984;33(8):1367-9. [PubMed ID: 6712740].
-
19.
Kimmel CA, Trammell C. A rapid procedure for routine double staining of cartilage and bone in fetal and adult animals. Stain Technol. 1981;56(5):271-3. [PubMed ID: 6171056].
-
20.
Soprano DR, Gyda M3, Jiang H, Harnish DC, Ugen K, Satre M, et al. A sustained elevation in retinoic acid receptor-beta 2 mRNA and protein occurs during retinoic acid-induced fetal dysmorphogenesis. Mech Dev. 1994;45(3):243-53. [PubMed ID: 8011556].
-
21.
Mulder GB, Manley N, Grant J, Schmidt K, Zeng W, Eckhoff C, et al. Effects of excess vitamin A on development of cranial neural crest-derived structures: a neonatal and embryologic study. Teratology. 2000;62(4):214-26. [PubMed ID: 10992263]. https://doi.org/10.1002/1096-9926(200010)62:4<214::AID-TERA7>3.0.CO;2-N.
-
22.
Kistler A. Teratogenesis of retinoic acid in rats: susceptible stages and suppression of retinoic acid-induced limb malformations by cycloheximide. Teratology. 1981;23(1):25-31. [PubMed ID: 7245088]. https://doi.org/10.1002/tera.1420230106.
-
23.
Tom C, Juriloff DM, Harris MJ. Studies of the effect of retinoic acid on anterior neural tube closure in mice genetically liable to exencephaly. Teratology. 1991;43(1):27-40. [PubMed ID: 2006470]. https://doi.org/10.1002/tera.1420430105.
-
24.
Dorko F, Spakovska T, Lovasova K, Patlevic P, Kluchova D. NADPH-d activity in rat thymus after the application of retinoid acid. Eur J Histochem. 2012;56(1). ee7. [PubMed ID: 22472895]. https://doi.org/10.4081/ejh.2012.e7.
-
25.
Emmanouil-Nikoloussi EN, Goret-Nicaise M, Foroglou CH, Katsarma E, Dhem A, Dourov N, et al. Craniofacial abnormalities induced by retinoic acid: a preliminary histological and scanning electron microscopic (SEM) study. Exp Toxicol Pathol. 2000;52(5):445-53. [PubMed ID: 11089896].
-
26.
Mahmood R, Flanders KC, Morriss-Kay GM. Interactions between retinoids and TGF beta s in mouse morphogenesis. Development. 1992;115(1):67-74. [PubMed ID: 1638993].
-
27.
Hansen LA, Pearl GS. Isotretinoin teratogenicity. Case report with neuropathologic findings. Acta Neuropathol. 1985;65(3-4):335-7. [PubMed ID: 3156465].
-
28.
Pauken CM, LaBorde JB, Bolon B. Retinoic acid acts during peri-implantational development to alter axial and brain formation. Anat Embryol (Berl). 1999;200(6):645-55. [PubMed ID: 10592067].
-
29.
Rutledge JC, Shourbaji AG, Hughes LA, Polifka JE, Cruz YP, Bishop JB, et al. Limb and lower-body duplications induced by retinoic acid in mice. Proc Natl Acad Sci U S A. 1994;91(12):5436-40. [PubMed ID: 8202504].
-
30.
Colakoglu N, Kukner A. Teratogenicity of retinoic acid and its effects on TGF-beta2 expression in the developing cerebral cortex of the rat. J Mol Histol. 2004;35(8-9):823-7. [PubMed ID: 15609095]. https://doi.org/10.1007/s10735-004-1683-y.
-
31.
Prater MR, Laudermilch CL, Liang C, Holladay SD. Placental oxidative stress alters expression of murine osteogenic genes and impairs fetal skeletal formation. Placenta. 2008;29(9):802-8. [PubMed ID: 18675455]. https://doi.org/10.1016/j.placenta.2008.06.010.
-
32.
Song Y, Liu J, Zhang F, Zhang J, Shi T, Zeng Z. Antioxidant effect of quercetin against acute spinal cord injury in rats and its correlation with the p38MAPK/iNOS signaling pathway. Life Sci. 2013;92(24-26):1215-21. [PubMed ID: 23688865]. https://doi.org/10.1016/j.lfs.2013.05.007.
-
33.
Gupta C, Vikram A, Tripathi DN, Ramarao P, Jena GB. Antioxidant and antimutagenic effect of quercetin against DEN induced hepatotoxicity in rat. Phytother Res. 2010;24(1):119-28. [PubMed ID: 19504466]. https://doi.org/10.1002/ptr.2883.
-
34.
Sanders RA, Rauscher FM, Watkins J3. Effects of quercetin on antioxidant defense in streptozotocin-induced diabetic rats. J Biochem Mol Toxicol. 2001;15(3):143-9. [PubMed ID: 11424224].
-
35.
Morales AI, Vicente-Sanchez C, Sandoval JM, Egido J, Mayoral P, Arevalo MA, et al. Protective effect of quercetin on experimental chronic cadmium nephrotoxicity in rats is based on its antioxidant properties. Food Chem Toxicol. 2006;44(12):2092-100. [PubMed ID: 16962696]. https://doi.org/10.1016/j.fct.2006.07.012.
-
36.
Perez-Pasten R, Martinez-Galero E, Chamorro-Cevallos G. Quercetin and naringenin reduce abnormal development of mouse embryos produced by hydroxyurea. J Pharm Pharmacol. 2010;62(8):1003-9. [PubMed ID: 20663034]. https://doi.org/10.1111/j.2042-7158.2010.01118.x.
-
37.
Liang C, Oest ME, Jones JC, Prater MR. Gestational high saturated fat diet alters C57BL/6 mouse perinatal skeletal formation. Birth Defects Res B Dev Reprod Toxicol. 2009;86(5):362-9. [PubMed ID: 19750487]. https://doi.org/10.1002/bdrb.20204.
-
38.
Abdelmoaty MA, Ibrahim MA, Ahmed NS, Abdelaziz MA. Confirmatory studies on the antioxidant and antidiabetic effect of quercetin in rats. Indian J Clin Biochem. 2010;25(2):188-92. [PubMed ID: 23105908]. https://doi.org/10.1007/s12291-010-0034-x.
-
39.
Dwivedi N, Flora SJS. Dose dependent efficacy of quercetin in preventing arsenic induced oxidative stress in rat blood and liver. J Cell Tissue Res. 2011;11(1):2505-611.