Abstract
Background:
The use of methamphetamine has been significantly increased among youth in the last decade. Methamphetamine stimulates the central nervous system and affects on the body tissues and neurotransmitters like dopamine. In this study, the effect of methamphetamine as a medicine of amphetamine group is investigated on the pituitary-gonad axis and spermatogenesis.Materials and Methods:
In this experimental study, 40 mature rats with 150±10 g weight are divided into in 4 groups including 10 rats in each group. Methamphetamine powder was weighted and it was solved in the normal saline to prepare a standard solution. Three experimental groups were injected in dosage 1, 3, 5 mg/kg using insulin syringe for 14 days, every day. Then, FSH, LH and testosterone concentrations were measured from the blood samples. The testis tissue was removed and after sectioning and staining by Hematoxylin-Eosin, was inspected by optical microscope for any changes. The average values of hormones and number of seminiferous cells were analyzed in SPSS-18 software using Duncan-test.Results:
Experimental results showed that by increasing the concentration of methamphetamine, the serum level of LH and testosterone increase. While the FSH concentration decrease by increasing the level of methamphetamine. Also, investigation on the testis tissue showed that spermatogenesis has decreased on it in comparison to control group. This is because of methamphetamine effect on the gonads.Conclusion:
The hormonal results and microscopic observations, it can conclude that using methamphetamine may cause destructive effects on pituitary-gonad axis and spermatogenesis and as a result, it may decrease fertility.Keywords
Introduction
Today, abusing psychogenic drugs is increasing among the young and the adolescents who are in the age of reproduction. Stimulus drugs have much more destructive effects and are harmful for the human’s health.
Narcotics are among of these materials (drugs) which were used from many years ago, and they may cause destructive effects on body. Of course, recently some medicines have been produced as psychogenic drugs which make energy and excitement and they force on destruction of dopaminergic and serotonergic neurons that may cause psychic severe dependency. Most of psychogenic drugs are of amphetamine derivatives and considered as stimulant medicines that have been made for clinical and medical purposes [1]. One of these drugs is methamphetamine that is known as glass or crystal in Iran. These drugs are classified as neurotoxin, because of harmful and destructive effect on central nervous system (CNS) [2]. The psychogenic drags have bad effects on heart, kidney, liver and the other parts of body. For example, endocrine system is not protected against to destructive effects of the drugs and they have many stimulant effects on pituitary - hypothalamus - thyroid and cause increasing the temperature of body [3]. Psychogenic drugs also increase the heartbeat [4]. In a scientific research, injecting methamphetamine to pregnant rats caused to harm the fetus heart muscle cells and made its growth abnormal [5]. Also, it is teratogenic and genetic poisonous effects have been studied by scientists [6, 7]. This abuse studied has many stimulating effects on pituitary hypothalamus-adrenal and cause to increase the secretion of adrnocorticotropic hormone (ACTH) and cortisone [8]. At present, various studies have investigated the effect of different materials such as pest controls, poisons, medicines and even cosmetics on fertilizing the male sex. In these studies other than testis, as a main part, more attention has been paid to the manner of sperm motion and mature in the path of epidigymis. As sperm gets a progressive motion and the capacity for inseminating ovule during passing in epididymis, the present materials in this channel are a proper environment to mature sperm [9, 10]. Regarding to the past researches, the aim of this research is studying the possible effects of methamphetamine on pituitary-gonad axis and spermatogenesis and probable. Effects depended to its rate. The researches in this field have been concentrated more on sexual behaviors and less to the effect of methamphetamine on the rate of sexual hormones. Therefore, in this study, the effect of methamphetamine on LH, FSH and testosterone and the spermatogenesis has been studied.
Materials and Methods
In this experimental study, 40 mature male rats with approximate weight 150±10 g and 3 months old were prepared from researching section of laboratory animals in Tehran Pastor Institute and transferred to Islamic Azad University of Falavarjan Branch. During the study, the animals had access to enough water and meal, and were kept in 26±2°C and 12 h light/darkness cycle. Methamphetamine crystalline powder was taken from the police organization in Isfahan. At first; the powder weighed and was saturated in the normal saline. The rats were divided into 4 groups consist of 10 rats (one control and 3 experiment groups).
Methamphetamine solution with 1, 3, 5 mg/kg concentrations were injected inter-peritoneum by an insulin syringe to 3 experimental groups on alternated days. The control groups were given experimental standard water and meal and no received any solution or medicine. The rats were weighted before injection and bleeding. After anesthesia, direct bleeding from heart was done. After analyzing the serum by RIA and hormonal kits FSH, LH and special kit of testosterone (Diaplus) made of Germany, the hormones were measured. Testis tissues were kept in a fixative 10% formalin solution and after coloring with Hematoxylin-Eosin were prepared to study by an optical microscope. The results due to the rate of hormones and counting sperm-maker cells were evaluated by unilateral variance analysis Duncan’s statistical test and p<0.05 was significant. Their related samples were drawn on Excel.
Results
The rat's weightiness results showed that the rats have been increased in all experiment groups, although it was not significant statistically (p<0.001). Plasma concentration of hormone LH (luteinizing hormone) was increased in compare to control group significantly, as its most rate was related to concentration 1 mg/kg (p<0.001) (Table 1). The rate of hormone FSH (follicle stimulating hormone) had significant viscosity reduction in comparing to control group (p<0.001) (Table 1).
Plasma concentration of testosterone hormone had significant increase against to control group, but it was not related to rate (of hormone) (p<0.001) (Table 1).
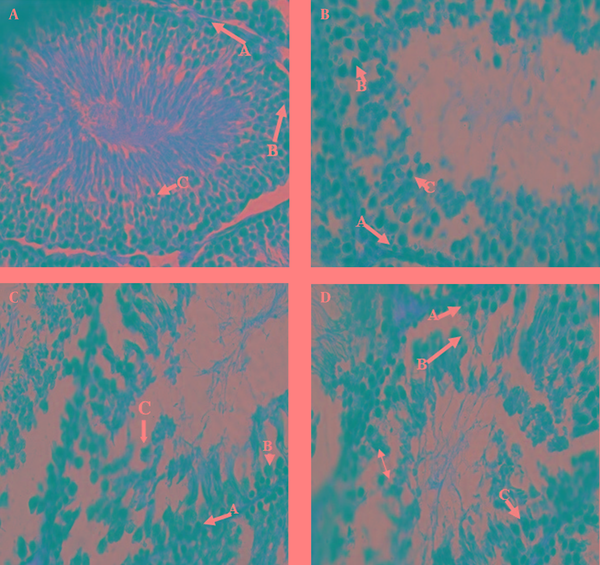
The prepared photomicrograph from cross-sectional of sperm-maker channels has been showed in figure 1-I (control groups), figure 1-II (experiment group with 1 mg/kg, figure 1-III (experiment group with 3 mg/kg) and figure 1-IV (experiment group with 5 mg/kg).
About 80 lam, 20 lam for each group and on each lam 3 sections from beginning, middle, and end of tissue were prepared to study different cells of testis tissue. The cells were identified under the optic microscope randomly and 15 data were determined in each group which was taken from tissue cross-sections [2].
According to table 2, comparing the cellular connting and figures show the transformations in the number of cells, spermatogonium core, primary spermatocyte and cells arrangement and also cell reproduction have been made that these transformations are related to the concentration and according to table 2, increasing the concentration of medicine, showed, a significant reduction in the average number of cells (p<0.001). But, there was no variation in cytoplasm property and their dye able (coloring). As it is showed in figure 1-IV, there is a little space between the layers of spermatogonium cells with primary spermatocyte and spermatid in some sperm-maker channels of experiment groups.
The mean serum testosterone, LH-FSH various experimental groups and the control group
| Control group Mean±SD | 1 mg/kg Mean±SD | 3 mg/kg Mean±SD | 5 mg/kg Mean±SD | p-Value | F | |
|---|---|---|---|---|---|---|
| Serum level of LH (IU/L) | 0.888±0.288 | 1.81±0.273 | 1.37±0.216 | 1.018±0.106 | 0.001 | 16.06 |
| Serum level of FSH (IU/L) | 1.54±0.23 | 1.07±0.0717 | 0.86±0.042 | 0.61±0.135 | 0.001 | 38.36 |
| Serum level of testosterone (ng/mL) | 0.592±0.186 | 1.192±0.152 | 2.246±0.731 | 1.148±0.238 | 0.001 | 14.69 |
Compared seminiferous cells in experimental groups to controls with different concentrations
| Control group Mean±SD | 1 mg/kg Mean±SD | 3 mg/kg Mean±SD | 5 mg/kg Mean±SD | p-Value | F | |
|---|---|---|---|---|---|---|
| Spermatogonium | 110.66±24.85 | 76.73±13.008 | 75.13±6.86 | 71.33±11.26 | 0.001 | 25.989 |
| Spermatocytes | 104±23.84 | 75.20±15.95 | 69.46±10.88 | 66.60±10.308 | 0.001 | 16.872 |
| Spermatids | 157.26±37.97 | 82.86±27.99 | 72.66±29.63 | 64.20±25.65 | 0.001 | 26.598 |
Seminiferous cells in control×400 staining H&E. II: Seminiferous cells in minimum dose groups (1mg/kg) drugs×400 staining H&E. III: Seminiferous cells in middling dose groups (3mg/kg) drugs×400 staining H&E. IV: Seminiferous cells in maximum dose groups (5mg/kg) drugs×400 staining H&E.
Discussion
In the present study, significant difference was not seen in control and experimental groups, weighs, before and after use of methamphetamine that it was opposed to our primary prehypothesis, because, the amphetamine derivates including methamphetamines have been recorded as an anti-appetite and leanness [11], but these results were corresponding to other findings. It is reported that despite of weigh loss in attendance groups in the first week of study, consuming the meal has increased in the second week [12]. Also, in another study, methamphetamine has been used as a decreasing factor of weight, while the amphetamine group reduces the appetite in a limited period and in following a long-term consumption may cause a resistance period against to this case [13]. In other study, it was showed that during a chronic stress (injecting medicine); it is more possible the rate of leptin secretion decrease [14]. Leptin hormone decreases the appetite and inverse the exogenous energy which will reduce the mass of fatness, and decrease in leptin hormone, cense to increase the weight.
Also, methamphetamine with inactive mutations in ob gene or db gene cause to decrease the leptin and increasing the weight which are corresponding to our results. In a natural condition of mature male rats, hypothalamus condition of mature male rats, hypothalamus and pituitary glands are responsible to control the secretion of testosterone and hormone secretion GnRH (Gonadotropin Releasing Hormone) by hypothalamus which helps to pituitary affects on leyding cells secrete LH and FSH with stimulant effect on supporting cells plays a role in transforming spermatid to sperm and spermatogenesis.
As it is observed in the results of hormone LH, the average concentration of LH showed a significant difference between experimental groups in comparing to control group and its rate has increased. These stimulant materials are effective on prolactin concentration and increase of prolactin can enhance the LH receptors [15]. In a research, it is showed that methylene deoxy- methamphetamine (MDMA) has more synthetic tendency with histamine H1 [16], and in other hand, histamine may increase the LH response to the secretion of GnRH (LHRH). But, it has no effect on the level base LH, and infect it causes to increase sensitiveness in receptors LH to the secretion of GnRH [17]. In Justo et al. study, it was identified that LH receptors are proteins and located on gonads that connect to the goal cells, testis leydig, that receptors Linked to AC (Aden late cyclase) and by increasing inter cellular CAMP may increase the rate of enzyme complex in mitochondria that cause to separate the cholesterol sub chain. The cholesterol changes into pregnenolone and the sexual hormone is made, and as a result, increasing the prolactin and then, increasing the number of receptors LH that may cause a variation in the rate of LH [18].
As in this study, the leydig cells have been destroyed and it helps to atrophy the testis, thus, by enhancing the rate of medicine and destroying the receptors LH, the rate of this hormone is decreased via increasing the drug concentration. As it is seen in the results of FSH, the rate of FSH has a significant reduction in comparing to control group. The results also show that methamphetamine Increased release of dopamine [19]. It was identified that neurotransmitter serotonin increased a little [20]. Serotonin may cause to increase the secretion of prolactin and it is followed the decrease in gonadotropin (FSH) [21]. de Kretser and Robertson studied the effect of stimulants on sexual hormones and it was recognized the high concentration of FSH can increase the glycoprotein and thus, in enhances inhibins which cause to decrease the rate of FSH in blood that they control each other as feedback [22]. Reduction in FSH is may due to feedback mechanism that it makes the hypothalamus-pituitary-autonom system axis, hyperactive [2]. The results showed that methamphetamine has bilateral effects. Regarding the finding in this study, a significant increase was observed in the rate of testosterone hormone of experimental groups, in peculiar, in 3 mg/kg concentration, against to the control group. In Scearce-Levie et al. study [23]. It was recognized that FSH cause to stimulate CAMP that it happens by protein kinas A, which stimulate gonadotropin and secretion the amphetamine effects on testosterone and its secretion from intermediate cells [24]. It was recognized that increasing circular AMP, decrease the calcium channels and the related enzymes to this hormone and by reducing the secretion of gonadotropins cause to decrease the secretion of testosterone [25]. Since intermediate cells are affected, by this stimulant, so in 5 mg/kg concentration, the rate of producing and secretion of testosterone will be less. Which is as same as researchers scientist so the studies show that methamphetamine decreases the testosterone concentration in rats and then it is increased in latter phases [26] Yamamoto et al. studied the effect of methamphetamine on sexual willing and it was recognized that this material reduces rats willing and ability for reproduction and in justification of this problem expressed that high rate of this abuse may reduce the sperm motion [26]. He used the TUNEL method (Tdt-mediated X-dUTPnick end labeling) and he studied the programmed cellular death induction in sperm maker channels in male rate by methamphetamine that it was with the variation of testosterone, but it was recognized that spermatogenic cells are very sensitive in comparing to neurons and even with the least rate, the apoptosis is increased [26]. That it is corresponding to these results. Testosterone and LH have interactive effect to each other are controlled by feedback [27]. CART (cocaine and amphetamine regulated transcript) is one of peptides in all parts of brain and in different concentrations of hypothalamus hormones [27]. CART level after care with cocaine or amphetamines will be increased [28]. It has been indentified that CART is one of these factors results in leaving GnRH (gonadotropin releasing hormone) and they are effective in encouragement, nutrition, stress activities and controlling endocrine [28, 29].
Nash et al. stated that methylen deoxy methamphetamine cause to destroy the serotonergic neurons in nervous system and make thermal variations and the body temperature is increased [30]. In rate of consumption 3-20 mg/kg, it increase the serum concentration of corticosterone and in 1-20 mg/kg, the prolactin concentration is increased which cause to vary testosterone level [31]. As it is seen, this variation is observable in 5 mg/kg concentration. According to table 1 it was identified by increasing the rate of drug, the number of cells and finally spermatids are reduced, that this reduction is due to apoptosis in spermatogenis cells in testis. In fact, due to apoptosis the number of cells in sperm channels will be redued [32]. In a study, it was identified with this procedure; the number of transferred sperms to the tail of epididymis will reduced. It is possible that by increasing the rate of drug, addition to induction of apoptosis cause to reduce the genetic cells. In this study, it was mentioned that hidden testis cause the rate of reproducing cells to apoptosis cells. But the result was opposed to that view; hidden testis can only cause the apoptosis in genetic cells of sperm [33].
In other research, the number of spermatogonium cells, spermatocyte and spermatid had been reduce, that one of its reason is due to apoptosis. Indeed, methamphetamine by reducing the reproductive cells and increasing apoptotic cells, may cause a reduction in reproductive cells against to apoptotic cell, thus it will disturb the natural homeostasis in testis [34]. In a study by Cadet et al. superoxide radicals are responsible for poisoning due to methylenedioximethamphetamine in nerves and tissues such as testis. One of the reasons for reduction in sexual cells is the existence of free radicals [35]. Although, there have been done many investigations the mechanism by which the reduction of cells is happened, is not clear, but male genitals system receives the adrenergic fibers and cholinergic from auto nervous system and neuroadrenergic fibers in male genitals system plays its role very well [36].
In other hand, methamphetamine cause to release [37] the mono amino neurotransmitters )noradrenalin, serotonin and dopamine) that it is possible methamphetamine by varying the rate of their secretion induces the mentioned effects. The results show that using methamphetamine can make destructive effects on pituitary gonad axis. Its long-term consumption other than destructive effects on the mentioned system by performance interventions on hormones also cause to increase LH and testosterone and reducing FSH. In the result of counting spermatogenesis cells it was observed that average of spermatogonium cells, spermatocytes and spermatid cells have had significant reduction in experimental group in comparing to control group and this reduction is related to the concentration that according to the researchers point of view. Abusing methamphetamine, even as amusing, can cause to disturb the natural homeostasis in testis by increasing the death of cells. Though the reduction of genetic cells of spermatogenesis is happened which its consequences are disability in pregnancy. In order to prove these cases, more investigations are necessary, especially in human samples.
Acknowledgements
References
-
1.
Faria R, Magalhaes A, Monteiro PR, Gomes-Da-Silva J, Amelia Tavares M, Summavielle T. MDMA in adolescent male rats: decreased serotonin in the amygdala and behavioral effects in the elevated plus-maze test. Ann N Y Acad Sci. 2006;1074:643-9. [PubMed ID: 17105959]. https://doi.org/10.1196/annals.1369.062.
-
2.
Hesami Z, Khatamsaz S, Mokhtari M. [The effects of ecstasy on pituitary - gonadal axis and spermatogenesis in mature male rats] Persian. Tabib-e-Shargh. 2008;10(3):207-18.
-
3.
Sprague JE, Banks ML, Cook VJ, Mills EM. Hypothalamic-pituitary-thyroid axis and sympathetic nervous system involvement in hyperthermia induced by 3,4-methylenedioxymethamphetamine (Ecstasy). J Pharmacol Exp Ther. 2003;305(1):159-66. [PubMed ID: 12649364]. https://doi.org/10.1124/jpet.102.044982.
-
4.
Henry BL, Minassian A, Perry W. Effect of methamphetamine dependence on heart rate variability. Addict Biol. 2012;17(3):648-58. [PubMed ID: 21182570]. https://doi.org/10.1111/j.1369-1600.2010.00270.x.
-
5.
Inoue H, Nakatome M, Terada M, Mizuno M, Ono R, Iino M, et al. Maternal methamphetamine administration during pregnancy influences on fetal rat heart development. [corrected]. Life Sci. 2004;74(12):1529-40. [PubMed ID: 14729402].
-
6.
Yamamoto Y, Yamamoto K. The teratogenicity of methamphetamine is influenced by housing conditions of pregnant mice. Congenit Anom. 1994;34(4):337-43. https://doi.org/10.1111/j.1741-4520.1994.tb00803.x.
-
7.
Yamamoto Y, Yamamoto K, Abiru H, Fukui Y, Shiota K. Effects of methamphetamine on rat embryos cultured in vitro. Biol Neonate. 1995;68(1):33-8. [PubMed ID: 7578635].
-
8.
Gerra G, Bassignana S, Zaimovic A, Moi G, Bussandri M, Caccavari R, et al. Hypothalamic-pituitary-adrenal axis responses to stress in subjects with 3,4-methylenedioxy-methamphetamine ('ecstasy') use history: correlation with dopamine receptor sensitivity. Psychiatry Res. 2003;120(2):115-24. [PubMed ID: 14527643].
-
9.
Lohiya NK, Mishra PK, Pathak N, Manivannan B, Bhande SS, Panneerdoss S, et al. Efficacy trial on the purified compounds of the seeds of Carica papaya for male contraception in albino rat. Reprod Toxicol. 2005;20(1):135-48. [PubMed ID: 15808797]. https://doi.org/10.1016/j.reprotox.2004.11.015.
-
10.
Srikanth V, Malini T, Arunakaran J, Govindarajulu P, Balasubramanian K. Effects of ethanol treatment on epididymal secretory products and sperm maturation in albino rats. J Pharmacol Exp Ther. 1999;288(2):509-15. [PubMed ID: 9918552].
-
11.
Climko RP, Roehrich H, Sweeney DR, Al-Razi J. Ecstacy: a review of MDMA and MDA. Int J Psychiatry Med. 1986;16(4):359-72. [PubMed ID: 2881902].
-
12.
Frith CH, Chang LW, Lattin DL, Walls RC, Hamm J, Doblin R. Toxicity of methylenedioxymethamphetamine (MDMA) in the dog and the rat. Fundam Appl Toxicol. 1987;9(1):110-9. [PubMed ID: 2887476].
-
13.
Shaw WN. Long-term treatment of obese Zucker rats with LY255582 and other appetite suppressants. Pharmacol Biochem Behav. 1993;46(3):653-9. [PubMed ID: 8278442].
-
14.
Heiman ML, Ahima RS, Craft LS, Schoner B, Stephens TW, Flier JS. Leptin inhibition of the hypothalamic-pituitary-adrenal axis in response to stress. Endocrinology. 1997;138(9):3859-63. [PubMed ID: 9275075]. https://doi.org/10.1210/endo.138.9.5366.
-
15.
McNeilly AS, Sharpe RM, Davidson DW, Fraser HM. Inhibition of gonadotrophin secretion by induced hyperprolactinaemia in the male rat. J Endocrinol. 1978;79(1):59-68. [PubMed ID: 361921].
-
16.
Fischer HS, Zernig G, Schatz DS, Humpel C, Saria A. MDMA ('ecstasy') enhances basal acetylcholine release in brain slices of the rat striatum. Eur J Neurosci. 2000;12(4):1385-90. [PubMed ID: 10762366].
-
17.
Knigge U, Wollesen F, Dejgaard A, Larsen K, Christiansen PM. Modulation of basal and LRH-stimulated gonadotrophin secretion by histamine in normal men. Neuroendocrinology. 1984;38(2):93-6. [PubMed ID: 6425709].
-
18.
Justo SN, Rossano GL, Szwarcfarb B, Rubio MC, Moguilevsky JA. Effect of serotoninergic system on FSH secretion in male and female rats: evidence for stimulatory and inhibitory actions. Neuroendocrinology. 1989;50(4):382-6. [PubMed ID: 2510047].
-
19.
Larsen KE, Fon EA, Hastings TG, Edwards RH, Sulzer D. Methamphetamine-induced degeneration of dopaminergic neurons involves autophagy and upregulation of dopamine synthesis. J Neurosci. 2002;22(20):8951-60. [PubMed ID: 12388602].
-
20.
Rudnick G, Wall SC. The molecular mechanism of "ecstasy" [3,4-methylenedioxy-methamphetamine (MDMA)]: serotonin transporters are targets for MDMA-induced serotonin release. Proc Natl Acad Sci U S A. 1992;89(5):1817-21. [PubMed ID: 1347426].
-
21.
Sharpe RM, McNeilly AS. The effect of induced hyperprolactinaemia on Leydig cell function and LH-induced loss of LH-receptors in the rat testis. Mol Cell Endocrinol. 1979;16(1):19-27. [PubMed ID: 227761].
-
22.
de Kretser DM, Robertson DM. The isolation and physiology of inhibin and related proteins. Biol Reprod. 1989;40(1):33-47. [PubMed ID: 2493821].
-
23.
Scearce-Levie K, Viswanathan SS, Hen R. Locomotor response to MDMA is attenuated in knockout mice lacking the 5-HT1B receptor. Psychopharmacology (Berl). 1999;141(2):154-61. [PubMed ID: 9952039].
-
24.
Kalra SP, Kalra PS. Neural regulation of luteinizing hormone secretion in the rat. Endocr Rev. 1983;4(4):311-51. [PubMed ID: 6360674]. https://doi.org/10.1210/edrv-4-4-311.
-
25.
Tsai SC, Chen JJ, Chiao YC, Lu CC, Lin H, Yeh JY, et al. The role of cyclic AMP production, calcium channel activation and enzyme activities in the inhibition of testosterone secretion by amphetamine. Br J Pharmacol. 1997;122(5):949-55. [PubMed ID: 9384514]. https://doi.org/10.1038/sj.bjp.0701463.
-
26.
Yamamoto Y, Yamamoto K, Hayase T. Effect of methamphetamine on male mice fertility. J Obstet Gynaecol Res. 1999;25(5):353-8. [PubMed ID: 10533332].
-
27.
Hurd YL, Fagergren P. Human cocaine- and amphetamine-regulated transcript (CART) mRNA is highly expressed in limbic- and sensory-related brain regions. J Comp Neurol. 2000;425(4):583-98. [PubMed ID: 10975881].
-
28.
Lebrethon MC, Vandersmissen E, Gerard A, Parent AS, Bourguignon JP. Cocaine and amphetamine-regulated-transcript peptide mediation of leptin stimulatory effect on the rat gonadotropin-releasing hormone pulse generator in vitro. J Neuroendocrinol. 2000;12(5):383-5. [PubMed ID: 10792575].
-
29.
Douglass J, McKinzie AA, Couceyro P. PCR differential display identifies a rat brain mRNA that is transcriptionally regulated by cocaine and amphetamine. J Neurosci. 1995;15(3 Pt 2):2471-81. [PubMed ID: 7891182].
-
30.
Nash JJ, Meltzer HY, Gudelsky GA. Elevation of serum prolactin and corticosterone concentrations in the rat after the administration of 3,4-methylenedioxymethamphetamine. J Pharmacol Exp Ther. 1988;245(3):873-9. [PubMed ID: 2898523].
-
31.
Tuomisto J, Mannisto P. Neurotransmitter regulation of anterior pituitary hormones. Pharmacol Rev. 1985;37(3):249-332. [PubMed ID: 2869509].
-
32.
Yamamoto Y, Yamamoto K, Hayase T, Abiru H, Shiota K, Mori C. Methamphetamine induces apoptosis in seminiferous tubules in male mice testis. Toxicol Appl Pharmacol. 2002;178(3):155-60. [PubMed ID: 11858731]. https://doi.org/10.1006/taap.2001.9330.
-
33.
Carmen M, Manas B, Morales E. Proliferation and apoptosis of spermatogonia inpostpuberal boar (susdomesticus) testes with spontaneous unilateral and bilateral abdominal cryptorchidism. Acta Histochem. 2005;107:365-72.
-
34.
Bernal-Manas CM, Morales E, Pastor LM, Pinart E, Bonet S, Rosa Pde L, et al. Proliferation and apoptosis of spermatogonia in postpuberal boar (Sus domesticus) testes with spontaneous unilateral and bilateral abdominal cryptorchidism. Acta Histochem. 2005;107(5):365-72. [PubMed ID: 16185749]. https://doi.org/10.1016/j.acthis.2005.07.002.
-
35.
Cadet JL, Sheng P, Ali S, Rothman R, Carlson E, Epstein C. Attenuation of methamphetamine-induced neurotoxicity in copper/zinc superoxide dismutase transgenic mice. J Neurochem. 1994;62(1):380-3. [PubMed ID: 7505315].
-
36.
Kempinas WD, Suarez JD, Roberts NL, Strader L, Ferrell J, Goldman JM, et al. Rat epididymal sperm quantity, quality, and transit time after guanethidine-induced sympathectomy. Biol Reprod. 1998;59(4):890-6. [PubMed ID: 9746740].
-
37.
Kalant H. The pharmacology and toxicology of "ecstasy" (MDMA) and related drugs. CMAJ. 2001;165(7):917-28. [PubMed ID: 11599334].