1. Background
Stress is defined as the state in which the brain interprets the quantity and quality of stimulation [1]. If the stress is intense or prolonged, the reaction to it follows three phases including alarm, resistance and exhaustion that lead to an adaptation of the body to the stress. Such adaptation can be the basis of some diseases such as cardiovascular disorders, psychiatric illnesses and also increases the susceptibility of the body to infection, autoimmune diseases, cancer, chronic fatigue and diabetes [2-4]. In humans, several studies have shown that stress may induce type 1 diabetes mellitus [5, 6]. Animal studies have also shown that different kinds of stressors may induce or inhibit type 1 diabetes in different experimental models of the disease [7]. Noise pollution is one of the harmful environmental factors for humans when it is higher than normal, could adversely affects to health [8, 9]. Noise as an environmental stress causes physiological and psychological changes in humans [10]. Studies have shown that chronic exposure to noise during pregnancy impairs neurobehavioral and reproductive functions and also reduces the body weight of the offspring. Also noisy environments cause stillbirths, fetal tratogenesis and abortion [11]. It seems that prenatal noise stress during last three months of fetal life damages the neurons in special areas of brain involved in cognition and impairs the activity of hypothalamus pituitary adrenal (HPA) axis. Among these cases can be pointed to the role of noise in diabetes. Because of cardiovascular risks due to hyperlipidemia, lipid disorders diagnosed and treated quickly as a part of comprehensive treatment of diabetes. The most common pattern of dyslipidemia is triglyceride elevation and HDL cholesterol reduction [12].
2. Objectives
Since the role of noise stress on histological changes of the pancreas and its function has not been investigated clearly yet, in this study the effects of noise stress on glucose levels, lipid profile and morphology of Langerhans islets in neonatal rat were investigated. The results of this study will be used in order to prevent diabetes especially type 1 to improve the level of serum factors and recovery of beta cells in the islets of Langerhans.
3. Materials and Methods
In this experimental study, 16 mature male rats with 225 ± 25 g weight have been purchased from Ahvaz Laboratory Animal Reproduction and Breeding Center. Rats have been separated to 2 groups with 8 members, randomly and each group was kept in 2 separate cages. In each cages two adult male rats were also kept. The animals were housed in an air-conditioned colony room on a 12/12 cycle at 23 - 27°C with 30 - 40% humidity and supplied with a standard pelleted diet and tap water ad libitum. Procedures involving animals and their care were conducted in the conformity with NIH guidelines for the care and use of laboratory animals.
For matching the menstrual cycle of female rats, intramuscular injection of estrogen valerate and a progestin (Abidi Pharmacy, Iran) were used. Then to detect estrus phase of rat, vaginal smear was prepared. Rats were in estrus phase was selected for research continue. Male rats were used for mating only. After vaginal plug of female rats were positive, they were separated. Stress noise induced group from the zero day of pregnancy until delivery were exposed to noise. Twelve weeks after delivery, also their babies were affected by noise stress. The control group with their newborn infants was initially kept in a separate room. After birth, 8 infants of both groups anesthetized with ether to measure blood factors at the first week, then with using the syringe blood samples were obtained from hearts. This procedure was repeated at 4th and 12th weeks respectively. Pancreas tissues were isolated for histological studies. Langerhans islands diameter, its distribution pattern and β cells condensation in Langerhans islands with Guemery’s staining method were calculated and compared in two mentioned groups.
3.1. Stress Procedure
One group of rats was exposed to white noise (90 - 120 db, 350 Hz). Exposure was started in the morning of first day for the period of 2 months, from 7 pm to 7 am intermittently (6 sound-hours per day). Each sound-hour consisted of a programmed variable intensity (intermittent noise) from low to high dB every 2 - 3 minutes by a noise generator device. The noise generator was off automatically after one hour and then restarted one hour later.
3.2. Measurement of Serum Glucose and Lipid Profile
Changes in body weight, food consumption and water intake were regularly observed during the experimental period. In addition, serum triglyceride, total cholesterol, and HDL cholesterol levels were spectrophotometrically measured using appropriate kits (Parsazmoon, Iran). LDL and very low density lipoprotein (VLDL) cholesterol levels were calculated by the following equation:
3.3. Panreas Histology
At the end of 12th week the rats were killed subsequent of total deep anesthesia and their pancreas gland tissue was isolated. The tissue after washing put in the normal saline and 10% formalin. Paraffin blocks were obtained after several tissue processing, and tissue section provides by microtome (Lyka, Germany) in 5 µm. The blocks were carried to lams and stained by Guemery’s staining method. At final the samples were observed by light microscope in 400 magnitudes. Langerhans islands diameter, its distribution pattern and β cells condensation in Langerhans islands were calculated and compared in two mentioned groups. For tissue assessment we Smart Sketch (version 3) software.
3.4. Statistics
Quantitative data were presented as Mean ± SEM. The data normality was checked using Kolmogorov-Smirnov test. For measurement at different times were used repeated measure ANOVA, while comparing data in different groups, t-test were used. In all states, P < 0.05 was regarded as significant difference. Data was evaluated by SPSS-12 for windows (SPSS Inc., Chicago, Illinois, USA).
4. Results
4.1. General Considerations
One rat was excluded at final weeks of the study due to severe weight loss and immobility. Meanwhile, 82% of rats were made diabetic following noise induction and had a serum glucose level higher than 250 mg/dL.
Body weight was measured in the 1st, 4th, 12th weeks in both groups. Results has shown that body weight were not significant in first week whereas some differences observed in 4th, 12th weeks. In control group a logical and natural increase in weight was observed in the 4th and 12th week. In noise induced group a weight loss was observed at twelfth week compared with the weights of the control group in the same week that was significant (P = 0.01). Serum glucose was measured in the 1st, 4th, 12th weeks in both groups. Results have shown that serum glucose was not significant in first week whereas some differences observed in 4th, 12th weeks. In addition, noise induced rats had also an elevated serum glucose level compare with control rats (P = 0.001). Results in control group were not significant.
Regarding serum lipids, noise induction after 12 weeks caused a significant increase in total cholesterol (P = 0.01), triglyceride (P = 0.05), LDL cholesterol (P = 0.005) and a significant reduction in HDL-cholesterol (P = 0.01) concentrations compared to baseline data (Table 1).
| Weight, g | Glucose, mg/dL | Total Cholesterol, mg/dL | Triglyceride, mg/dL | HDL, mg/dL | LDL, mg/dL | |
|---|---|---|---|---|---|---|
| First week | 11.2 ± 2.5 | 141.7 ± 9.8 | 75.1 ± 3.5 | 67.2 ± 4.9 | 41.1 ± 2.4 | 20.5 ± 1.9 |
| Fourth week | 58.6 ± 5.1 | 159 ± 11.9 | 79.2 ± 4.1 | 68.6 ± 4.2 | 42.7 ± 2.3 | 22.7 ± 2.2 |
| Twelfth week | 206.5 ± 10.2 | 141.4 ± 7.9 | 78.6 ± 4.7 | 64.4 ± 4.3 | 42 ± 2 | 23.7 ± 2.3 |
| First week | 11.2 ± 3.6 | 127.8 ± 12.9 | 75.8 ± 3.7 | 64.9 ± 4.3 | 38.1 ± 2.2 | 24.7 ± 2.1 |
| Fourth week | 44.6 ± 6.8 | 378 ± 23.4 | 90.9 ± 4.3 | 102.3 ± 5.4 | 24.7 ± 2.5 | 45.7 ± 2.6 |
| Twelfth week | 173 ± 11.4 | 401 ± 25.9 | 94.7 ± 4.5 | 104.1 ± 5.5 | 25.9 ± 2.5 | 47.9 ± 2.9 |
aValues are presented as mean ± SD.
bP = 0.01 (as compared to week 0 in the same group).
cP = 0.001 (as compared to week 0 in the same group).
dP = 0.05 (as compared to week 0 in the same group).
eP = 0.005 (as compared to week 0 in the same group).
Meanwhile, comparing control and noise induced groups showed that there were significant differences between them after 12 weeks regarding serum triglyceride, HDL- and LDL cholesterol level.
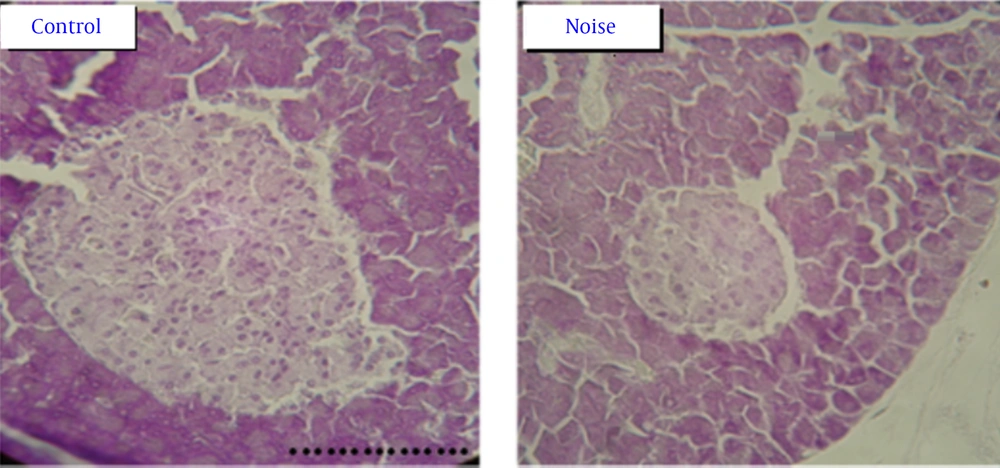
Histology of pancreas gland: The pancreas tissue in control group have Langerhans islands with well borders and beta cells with clear margins and excretory granules are abundant, but in noise induced diabetic rats have granules with atrophic and wrinkle changes. In addition, the β cells were degranulated and were diminished. In noise induced diabetic rats the number of β cells in each island was significant decrease comparing β cells in control group (Table 2 and Figure 1).
| Group | Langerhans Islands Environments, µm |
|---|---|
| 475.3 ± 16.5 | |
| 236.4 ± 19.4b |
aValues are presented as mean ± SD.
bP = 0.001 (as compared to control group in twelfth week).
5. Discussion
In this study, development of diabetic indicators in noise stress-induced rats was confirmed after three months. The results of this study demonstrate that fasting plasma glucose levels were increased on the 4th and 12th weeks of the experiment in the stressed group as compared to the control group. Also LDL cholesterol levels were increased on the 4th and 12th weeks of the experiment in the stressed group as compared to the control group but HDL cholesterol levels were decreased on these times of the experiment in the stressed group as compared to the control group. Our results also have shown that chronic noise stress caused significantly lower body weight in stressed rats, on the 4th and 12th weeks of the experiment. Also histology of Langerhans islets showed a lower number and granularity of beta cells in the stressed group as compared to the control group.
Diabetic induce condition by some materials like streptozotocin, alloxan in rodent like rat is associated with degenerative changes in the pancreas island of Langerhans and prominent and undesirable changes in the lipid serum and lipoprotein level of the plasma. According to this, some tissues like liver have essential roles in absorption of blood free fatty acid, oxidation and metabolic exchange to other material, cholesterol, phospholipids exceeding and exertion of some kind of lipoprotein into the blood. According to previous research finding in diabetic rats induced by alloxan or streptozotocin, increase in blood glucose level [13] in directly lead to increase in serum cholestrol, TG, LDL, VLDL and decrease HDL cholesterol serum level [14]. These finding confirm that undesirable changes of lipid serum levels are exist in noise induced diabetic rats in this study. Increased glucose levels observed during stress could be the combined result of two mechanisms, the hyperglycemic effect of catecholamines and glucocorticoids, released by the activation of the sympatho-adreno-medullary and pituitary-adreno-cortical systems, respectively [15-17] and, a second mechanism, the α-adrenergic inhibition of insulin secretion through activation of the sympatho-adreno-medullary system [17-19]. On the other hand, stress hormones such as the growth hormone and glucocorticoids not only increase plasma glucose concentration but also have a stimulatory effect on pancreatic β cells, possibly by enhancing sensitivity to glucose, and also indirectly stimulate insulin secretion by inducing a state of insulin resistance [18, 19].
Our results also have shown that chronic noise stress caused significantly lower body weight in stressed rats, on the 4th and 12th weeks of the experiment. Increasing the activity of corticotropin releasing hormone (CRH), as an anorexigenic neuropeptide, subsequent to stress exposure, led to a reduction of food intake and bodyweight in the stressed rats [20, 21]. In the chronically stressed rats, even if food intake is not altered or increased, a reduction in body weight gain is observed. This result may be due to stress activation of the rich sympathetic innervations of brown adipose tissue, which is possibly increased in stressed rats [22, 23].
In conclusion, the results of the present study demonstrate that chronic noise stress increases fasting plasma glucose and lipid profile and decrease body weight levels and degenerates pancreas tissue over 90 days in noie group. The most interesting finding of this study is that pancreatic β cells from rats stressed for 90 days demonstrate decreased intensively compared to controls. However, this finding should be considered in future studies on the destructive effects of noise stress.