Abstract
Background:
Candida spp. has been considered as the agents of acute and recurrent vulvovaginal candidiasis.Objectives:
The aim of current study was the evaluation of antifungal activity of Echinops cephalotes (Leaves and stem, manna) plant against species of Candida isolated from patients with vulvovaginal candidiasis.Materials and Methods:
In this research study identification of clinical isolates (50 cases) was inducted to the species level by means of conventional mycological methods, morophology on corn meal agar and chromogenic agar, germ tube production and biochemical methods. Antifungal activity of the ethanolic, methanolic and aqueous extracts of E. cephalotes was studied against isolated Candida using agar well diffusion and microdilution methods.Results:
Candida spp. which isolated from patients was C. albicans, C. glabrata, C. tropicalis and C. parapsilosis. The inhibition zone of ethanolic extract was 16.6, 13.3, 14, and 22 mg/mL respectively. Minimum inhibitory concentration (MIC) for most the cases were 15.6 mg/mL. The inhibition zone of aqueous extract was 16.8, 16.7, 15 and 15 mg/mL respectively. MIC for most the cases were 15.6-31.2 mg/mL. The inhibition zone of methanolic extract was 15.4, 13.2, 12 and 18 respectively. MIC for most of the cases was 7.8 mg/mL. Among the different extracts, ethanolic extract has the highest and aqueous extract has the lowest anti-Candida activity. Ethanolic, methanolic and aqueous extracts of trehala manna did not show any antifungal activity.Conclusions:
This research is the first study on antifungal activity of E. cephalotes. Hence, this plant may be used further as medicinal plant against Candida spp.Keywords
Vulvovaginal Candidiasis Extract Echinops cephalotes Candida
1. Background
Candida species have been introduced as commensal fungi which are the most common cause of invasive fungal infections in humans [1]. In healthy individuals Candida species are harmless members of the normal gastrointestinal, oral and vaginal microbial flora [2]. Candidiasis is an opportunistic fungal infection caused by many species of Candida that may affects different sites of the body [3]. Fungal infections also occur in chronic illnesses like diabetes and AIDS [4]. Vulvovaginal candidiasis (VVC) is a disease caused by overgrowing of yeasts in the mucosa of the female genital tract [2]. Approximately 75% of adult women will experience at least one episode of VVC during their lifetime, among which approximately 40 - 50% will experience further episodes and 5% will develop the recurrent type (RVVC) [5]. The majority of cases of vulvovaginal candidiasis are caused by C. albicans; however, non-albicans species such as C. tropicalis, C. glabrata, C. krusei and C. parapsilosis are common in certain populations [5]. A possible reason for more frequent isolation of non-albicans species from vulvovaginitis patients may be related with the overuse of topical azole agents available as over-the-counter preparations in the United States since 1992 [6]. This phenomenon emphasizes the importance of identification of the Candida species in the clinical settings. Herbal medicines have been used since ancient times as remedies for the treatment of a variety of diseases. In spite of advances in modern medication, plants still make an important contribution to health care [7]. Herbal extracts prepared from medicinal plants include different compounds with numerous biological activities confirmed by in vitro and in vivo studies, such as antibacterial, antifungal, antiviral, antiseptic, anti-inflammatory, antitumor, anti oxidative, anti allergic and diuretic properties [8].
The frequency of invasive fungal infections in one hand and resistance to antifungal therapy in other hand may lead to introduce new antifungal agents. In vitro antifungal susceptibility testing is now standardized internationally and is becoming essential in patient management and resistance surveillance [9]. Traditional plants are valuable source of novel antifungal [10]. E. cephalotes (Trehala manna), is product of the hard work on insect of Larinus spp. on the species of Echinops. The genus belonging to the family of Compositae species. Echinops genus of Asteraceae family has 120 species distributed all over the world and according to Flora Iranica there are 54 species in different area of Iran. There is an exception of four Larinus species constructing trehala, whose larvae make capsule in the stems of the plant genus Echinops. The results showed that, although the Larinus insect was seen on the all of the species, they produce manna, only on four species, (E. endotrichus, E. dichrous, E. tenuisectus and E. persepolitanus). Morphologaical investigations indicated that manna produce species have spherical aggregated flowers and they have the same plant height [11, 12].
2. Objectives
The aim of this study was the investigation of inhibitory effect of Echinops cephalotes (Leaves and stem, manna) on Candida spp. isolated from vulvovaginal candidiasis patients.
3. Materials and Methods
In this research study the aerial parts of plant were collected in spring 2011 from Ardestan in Isfahan province. The collected plants were identified in the agricultural and natural resources of the research center of Isfahan, Iran.
3.1. Preparation of Alcoholic and Aqueous Extracts
Leaves and stems of E. cephalotes were dried, powdered and stored in 4°C for further investigations. Fifty grams powdered plant materials were extracted in a soxhlet apparatus with 270 mL of each of solvent namely ethanol, methanol and aqueous for 8 hours, the alcoholic and aqueous extracts were evaporated at room temperature. Then 1gr of the dried plant extract was dissolved in 4 mL dimethylsulfoxide (DMSO) to obtain a final concentration of 250 mg/mL. Serial dilutions were performed, the extracts were tested at six concentrations that varied from 250 to 7.8 mg/mL [13].
3.2. Microorganisms and Identification
Clinical isolates of pathogenic yeast were obtained from 50 patients who visited in the pathological laboratory of Dr. Vahabi and Gharazi hospital in Isfahan for examinations of vaginal secretion. Randomly samples were selected on the basis of similar clinical. Cervical and vaginal specimens for the culture were collected with the aid of a disposable vaginal speculum (Vagispec, Brazil) and immediately spread on sabouraud dextrose agar medium «SDA» (Shaurlo, Spania) supplemented with 50 mg/mL of chloramphenicol (Sigma, USA). The dishes were incubated at 37°C for 48 - 72 hours, then a pool of the grown colonies were subcultured in CHROM agar Candida (CHROMagar Candida, France) to investigate the purity of the culture and the color of the colonies [14]. From this selective and differential medium the yeasts were identified according to biochemical tests and standard procedures including morphology, germ tube test and corn meal agar test [5].
3.3. Preparation of Inoculum
The fungal suspensions were prepared as per 0.5 McFarland turbidity standards. A 24 hours old culture was used for the preparation of fungal suspension. To prepare the inocula, yeast cell suspensions were adjusted such that it contained approximately 1.5 × 106 CFU/mL [15]. standard Mcfarland 0.5 containing barium chloride and sulphoric acid was used as turbidity control [16].
3.4. Antifungal Susceptibility Testing
The antifungal activities of extracts against Candida spp. isolated from patient with vulvovaginal candidiasis were determined using agar diffusion and micro-dilution methods. So Clinical and laboratories standards institute (CLSI) were used for measuring in vitro susceptibility of microorganisms to antimicrobial agents used in clinical settings [17].
3.5. Agar Diffusion Assay:
Twenty microliter of each test yeasts regularly was spread using a sterile glass spreader onto Sabouraud dextrose agar plates. The plates have been kept to dry and a sterile borer (6.4 mm in diameter) then used to punch holes in the agar medium. Subsequently, holes were filled with 150 μL of the plant extract at concentration of 7.8 - 250 mg/mL and allowed to diffuse at room temperature for 2 hours. The plates were incubated at 25°C for 48 hours. The experiments were triplicate and the mean of the inhibition zone of each tested fungi was measured. Diameters less than 5 mm indicated no effect [10]. Sterile DMSO used as negative control. Fluconazole was used in the assay as positive control.
3.6. Microdilution Assay
The minimum inhibitory concentration (MIC) determination was carried out according to the national committee for clinical laboratory standards institute (M27-A) guidelines [18]. Extracts were dissolved in 100 μL sabouraud dextrose broth «SDB» (Shaurlo, Spania). The extracts were tested at six concentrations into sabouraud dextrose broth that varied from 7.8 to 250 mg/mL. Microdilution method performed in sterile 96-well microplates. From each dilution, 100 μL volumes were distributed in microplates. All the wells were then filled with 10 μL of stock yeast culture. The following control wells were parallel prepared: wells contain only broth; other wells contain Candida strain without extract, and serial dilutions of fluconazole with the fungi at the recommended inhibitory concentrations. Absorbance was read at 630 nm, then wells were covered with sterile parafilm, and incubated at 35°C for 24 - 48 hours. Absorbance was reread at the same wave length [13, 19]. All tests were performed in triplicate. MICs are defined as the lowest drug concentration of the extract at which there was no visible growth of organisms [20].
3.7. Determination of the MFC (Minimum Fungicidal Concentration)
The MFC of E. cephalotes for the 50 strains were determined as described previously. Briefly, after the MIC for each strain was determined, 20 μL suspensions from each well of microtiter plates that prevented the visible growth of a microorganism were subcultured onto sabouraud dextrose agar plates. The MFC was defined as the lowest drug concentration at which less than three fungal colonies were observed after 48 hours of incubation at 35°C [21]. Statistical analysis was performed using SPSS-18 software.
The results at the level of P < 0.001 were analyzed. We observe ethics in various stages of research, particularly clinical research.
4. Results
Candida spp. isolated from 50 patients with vulvovaginal candidiasis was C. albicans (50%), C. glabrata (46%), C.parapsilosis (2%), and C.tropicalis (2%). The antifungal activity was determined by measuring the diameter of zone of inhibition recorded [22]. The results are as shown in Figures 1 and 2.
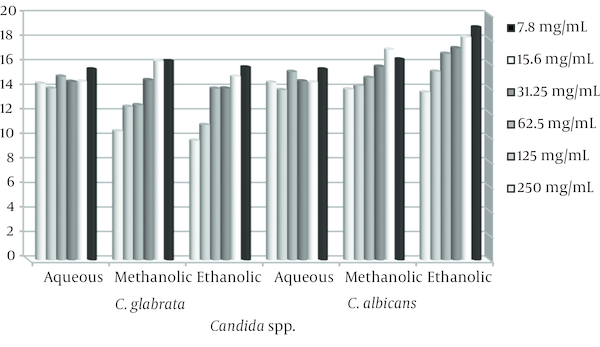
Average Inhibition Zone of Ethanolic, Methanolic and Aqueous Extracts Against C. albicans and C. glabrata
Average Inhibition Zone of Ethanolic, methanolic and Aqueous Extracts Against C. parapsilosis and C. tropicalis
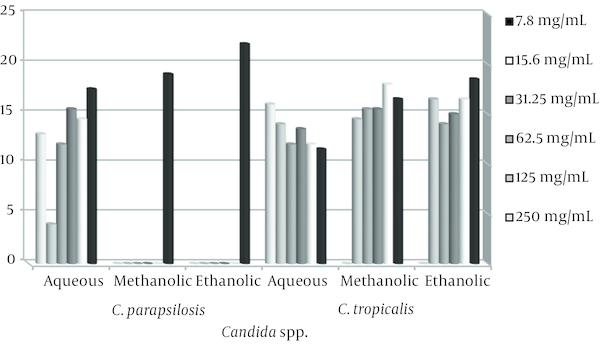
The stem and leaves extracts of E. cephalotes revealed strong activity with a 10 - 36 mm zone of inhibition against Candida spp. Alcoholic extracts of the plant were found to have maximum antifungal activity in comparison to aqueous extract. Ethanolic extract of E. cephalotes showed similar antifungal activity against C. albicans showing zone of inhibition viz. 35.5 mm (Table 1). Methanolic extract of E. cephalotes possessed potent antifungal activity among all the extracts against C. albicans showing zone of inhibition viz. 36 mm (Table 2). Aqueous extracts of E. cephalotes showed antifungal activity against C. albicans with diameter of inhibition zone of grow viz. 30.5 mm (Table 3). While ethanolic and aqueous extract showed stronger antifungal activity comparison to methanolic extract against C. glabrata. The results of the inhibition zone of grow the ethanolic, methanolic and aqueous of E. cephalotes extracts against C. glabrata are shown in tables 4, 5 and 6. In C. tropicalis isolate inhibition zone of grow the ethanolic and methanolic extracts in the range of 14 - 18 mm and aqueous extract in the range 11 - 16 mm respectively, as a result antifungal activity of ethanolic and methanolic extracts compared to aqueous extract is better. For C .parapsilosis in high concentrations of ethanolic and methanolic extracts, inhibition zone of grow is not show but in the concentration of 7.8 mg/mL zone of inhibition equivalent 22 mm was created, and inhibition zone of the aqueous extract in different concentrations of 12 - 17.5 mm was variable.
Antifungal Activity of Ethanolic Extract of E. cephalotes Against C. albicans
| Con, mg/mL | 7.8 | 15.6 | 31.25 | 62.5 | 125 | 250 | MIC | MFC |
|---|---|---|---|---|---|---|---|---|
| Inhibition Zone, mm | ||||||||
| Ca1 | 13.5 | 14.5 | 15 | 15.5 | 16.5 | 19 | 15.6 | 31.2 |
| Ca2 | 19 | 19.5 | 17 | 13.5 | 14 | 14 | 15.6 | 31.2 |
| Ca3 | 11.5 | 13 | 14 | 14 | 14 | 14 | 15.6 | 31.2 |
| Ca4 | 29 | 14 | 13 | 25 | 24 | 20.5 | 31.2 | 62.5 |
| Ca5 | 32.5 | 17.5 | 20.5 | 18.5 | 16 | 0 | 31.2 | 62.5 |
| Ca6 | 13.5 | 21 | 14 | 15.5 | 13 | 11.5 | 7.8 | 15.6 |
| Ca7 | 25 | 22.5 | 21.5 | 20.5 | 18.5 | 18 | 7.8 | 15.6 |
| Ca8 | 16 | 17.5 | 15 | 14.5 | 12.5 | 12 | 15.6 | 31.2 |
| Ca9 | 19.5 | 16 | 14.5 | 11.5 | 11 | 11 | 15.6 | 31.2 |
| Ca10 | 13.5 | 12.5 | 5 | 0 | 0 | 0 | 15.6 | 31.2 |
| Ca11 | 34.5 | 26.5 | 22 | 17.5 | 15.5 | 15 | 15.6 | 31.2 |
| Ca12 | 21 | 19.5 | 18 | 15 | 15 | 15.5 | 15.6 | 31.2 |
| Ca13 | 0 | 0 | 0 | 13.5 | 14 | 15 | 15.6 | 31.2 |
| Ca14 | 26 | 23 | 22 | 19 | 17 | 16.5 | 15.6 | 31.2 |
| Ca15 | 0 | 0 | 0 | 11.5 | 11.5 | 15.5 | 15.6 | 31.2 |
| Ca16 | 25.5 | 30.5 | 28.5 | 26 | 25.5 | 26.5 | 15.6 | 31.2 |
| Ca17 | 21 | 26 | 25.5 | 21 | 16.5 | 15 | 15.6 | 31.2 |
| Ca18 | 20.5 | 19.5 | 18.5 | 15.5 | 11.5 | 10.5 | 15.6 | 31.2 |
| Ca19 | 36 | 36 | 35.5 | 28.5 | 27 | 26.5 | 15.6 | 31.2 |
| Ca20 | 25.5 | 21 | 20 | 17.5 | 15.5 | 0 | 15.6 | 31.2 |
| Ca21 | 14.5 | 16.5 | 15.5 | 17.5 | 13 | 13.5 | 15.6 | 31.2 |
| Ca22 | 14.5 | 22.5 | 29.5 | 24 | 16 | 13.5 | 31.2 | 62.5 |
| Ca23 | 12.5 | 12 | 16 | 16.5 | 18.5 | 15 | 15.6 | 1.2 |
| Ca24 | 15.5 | 15.5 | 15 | 14 | 14.5 | 11.5 | 15.6 | 31.2 |
| Ca25 | 14.5 | 17.5 | 17 | 15 | 14 | 12.5 | 15.6 | 31.2 |
| Ave | 18.9 | 18.1 | 17.3 | 16.8 | 15.3 | 13.6 | 16.8 | 32.5 |
Antifungal Activity of Methanolic Extract of E. cephalotes Against C. albicans
| Con, mg/mL | 7.8 | 15.6 | 31.25 | 62.5 | 125 | 250 | MIC | MFC |
|---|---|---|---|---|---|---|---|---|
| Inhibition Zone, mm | ||||||||
| Ca1 | 24 | 12 | 13 | 14 | 15 | 17.5 | 7.8 | 15.6 |
| Ca2 | 16.5 | 18 | 15.5 | 13 | 13 | 13.5 | 7.8 | 15.6 |
| Ca3 | 26 | 11 | 12 | 12.5 | 12.5 | 12.5 | 7.8 | 15.6 |
| Ca4 | 12.5 | 17 | 17.5 | 15.5 | 24.5 | 20.5 | 7.8 | 15.6 |
| Ca5 | 14.5 | 17.5 | 15.5 | 0 | 0 | 0 | 7.8 | 15.6 |
| Ca6 | 19 | 19 | 22 | 18.5 | 18 | 15 | >7/8 | 7.8 |
| Ca7 | 13 | 24.5 | 22.5 | 20.5 | 20 | 20 | 7.8 | 15.6 |
| Ca8 | 24 | 20 | 18.5 | 16.5 | 14.5 | 13 | 7.8 | 15.6 |
| Ca9 | 11.5 | 12.5 | 13 | 18 | 17.5 | 18 | 7.8 | 15.6 |
| Ca10 | 19 | 14 | 0 | 0 | 0 | 0 | 7.8 | 15.6 |
| Ca11 | 24 | 20 | 20.5 | 16 | 5 | 14 | 7.8 | 15.6 |
| Ca12 | 12 | 18 | 15 | 18 | 16 | 16.5 | 7.8 | 15.6 |
| Ca13 | 11.5 | 0 | 0 | 15.5 | 16.5 | 8.5 | 7.8 | 15.6 |
| Ca14 | 14 | 23 | 22.5 | 19.5 | 15.5 | 20 | 7.8 | 15.6 |
| Ca15 | 15 | 0 | 0 | 16 | 14 | 13 | 15.6 | 31.2 |
| Ca16 | 16.5 | 30.5 | 30.5 | 25.5 | 25 | 26.5 | 31.2 | 62.5 |
| Ca17 | 15 | 27.5 | 24 | 19.5 | 19 | 20.5 | 7.8 | 15.6 |
| Ca18 | 12.5 | 16 | 16 | 15 | 12 | 12.5 | 7.8 | 15.6 |
| Ca19 | 14.5 | 36 | 35.5 | 22.5 | 26 | 26 | 7.8 | 15.6 |
| Ca20 | 12.5 | 6.5 | 0 | 0 | 0 | 0 | 15.6 | 31 |
| Ca21 | 18.5 | 21.5 | 18.5 | 19.5 | 19 | 19.5 | 7.8 | 15.6 |
| Ca22 | 12 | 18.5 | 18 | 13 | 13 | 14 | 15.6 | 31.2 |
| Ca23 | 22.5 | 14 | 12.5 | 15.5 | 15 | 14.5 | 15.6 | 31.2 |
| Ca24 | 11.5 | 16 | 16.5 | 15.5 | 11 | 0 | 15.6 | 31.2 |
| Ca25 | 17 | 16.5 | 15.5 | 12.5 | 13 | 12.5 | 7.8 | 15.6 |
| Ave | 16.3 | 17.1 | 15.7 | 14.8 | 14.2 | 13.9 | 10.4 | 20.2 |
Antifungal Activity of Aqueous Extract E. cephalotes Against C. albicans
| Con(mg/mL) | 7.8 | 15.6 | 31.25 | 62.5 | 125 | 250 | MIC | MFC |
|---|---|---|---|---|---|---|---|---|
| Inhibition Zone, mm | ||||||||
| Ca1 | 15.5 | 14.5 | 17 | 16 | 4.5 | 15.5 | 15.6 | 31.2 |
| Ca2 | 19 | 16 | 19.5 | 17.5 | 14.5 | 12 | 7.8 | 15.6 |
| Ca3 | 21 | 21.5 | 24.5 | 26 | 21.5 | 25.5 | 7.8 | 15.6 |
| Ca4 | 18.5 | 19.5 | 19 | 21 | 19.5 | 18.5 | 15.6 | 31.2 |
| Ca5 | 22.5 | 22.5 | 19.5 | 19.5 | 21.5 | 22 | 7.8 | 15.6 |
| Ca6 | 16.5 | 19 | 21.5 | 19.5 | 15.5 | 15 | <7.8 | 7.8 |
| Ca7 | 21 | 22.5 | 20 | 20.5 | 17.5 | 15.5 | 7.8 | 15.6 |
| Ca8 | 13.5 | 13.5 | 14.5 | 20.5 | 20.5 | 21 | 31.2 | 62.5 |
| Ca9 | 22 | 15 | 18 | 16.5 | 14.5 | 20.5 | 7.8 | 15.6 |
| Ca10 | 20 | 16.5 | 15.5 | 0 | 0 | 0 | 7.8 | 15.6 |
| Ca11 | 25.5 | 23.5 | 21.5 | 18 | 19.5 | 21 | 7.8 | 15.6 |
| Ca12 | 24 | 21.5 | 25.5 | 21.5 | 20 | 20 | 15.6 | 31.2 |
| Ca13 | 13 | 12.5 | 12.5 | 17.5 | 0 | 0 | 15.6 | 31.2 |
| Ca14 | 31 | 30.5 | 30.5 | 26 | 25.5 | 24 | 15.6 | 31.2 |
| Ca15 | 17 | 17.5 | 15.5 | 21.5 | 16.5 | 12 | 15.6 | 31.2 |
| Ca16 | 27.5 | 30.5 | 21 | 21.5 | 21.5 | 25.5 | 15.6 | 31.2 |
| Ca17 | 15.5 | 18 | 19.5 | 26.5 | 21 | 20.5 | 15.6 | 31.2 |
| Ca18 | 15.5 | 15.5 | 15 | 11.5 | 20.5 | 23 | 15.6 | 31.2 |
| Ca19 | 14.5 | 15.5 | 15.5 | 14.5 | 14.5 | 16 | 31.2 | 62.5 |
| Ca20 | 17 | 17 | 16.5 | 7.5 | 18 | 13 | 31.2 | 62.5 |
| Ca21 | 14.5 | 16.5 | 14.5 | 15.5 | 14.5 | 12 | 31.2 | 62.5 |
| Ca22 | 19.5 | 14.5 | 19 | 0 | 0 | 0 | 31.2 | 62.5 |
| Ca23 | 0 | 5 | 3.5 | 17 | 11 | 14.5 | 31.2 | 62.5 |
| Ca24 | 15.5 | 14.5 | 14.5 | 11.5 | 16 | 16 | 31.2 | 62.5 |
| Ca25 | 10.5 | 12.5 | 11.5 | 10 | 9.5 | 0 | 31.2 | 62.5 |
| Ave | 18 | 17.8 | 17.8 | 16.6 | 15.1 | 15.3 | 18.5 | 35.9 |
Antifungal Activity of Ethanolic Extract of E. cephalotes against C. glabrata
| Con, mg/mL | 7.8 | 15.6 | 31.25 | 62.5 | 125 | 250 | MIC | MFC |
|---|---|---|---|---|---|---|---|---|
| Inhibition Zone, mm | ||||||||
| Cg1 | 24.5 | 21.5 | 21 | 23.5 | 19 | 21 | 15.6 | 31.2 |
| Cg2 | 25.5 | 20.5 | 18.5 | 18 | 16.5 | 16 | 15.6 | 31.2 |
| Cg3 | 10 | 9.5 | 0 | 0 | 0 | 0 | 15.6 | 31.2 |
| Cg4 | 13 | 13 | 14 | 15 | 16 | 16 | 15.6 | 31.2 |
| Cg5 | 13.5 | 10.5 | 0 | 0 | 0 | 0 | 7.8 | 15.6 |
| Cg6 | 24.5 | 21.5 | 22 | 21 | 10 | 16 | 7.8 | 15.6 |
| Cg7 | 14 | 11.5 | 12 | 11 | 10 | 0 | 31.2 | 62.5 |
| Cg8 | 24 | 21.5 | 21.5 | 20 | 13.5 | 12.5 | 15.6 | 31.2 |
| Cg9 | 23.5 | 22 | 19 | 15.5 | 11 | 12.5 | 31.2 | 62.5 |
| Cg10 | 14 | 15 | 15.5 | 17.5 | 15.5 | 17.5 | 15.6 | 31.2 |
| Cg11 | 5.5 | 10 | 15.5 | 16.5 | 0 | 0 | 15.6 | 31.2 |
| Cg12 | 14 | 14.5 | 13.5 | 12 | 12.5 | 10.5 | 7.8 | 15.6 |
| Cg13 | 13.5 | 10.5 | 10 | 0 | 0 | 0 | 15.6 | 31.2 |
| Cg14 | 17.5 | 17.5 | 13.5 | 13 | 10 | 0 | 15.6 | 31.2 |
| Cg15 | 14.5 | 15.5 | 14 | 15.1 | 18.5 | 16 | 15.6 | 31.2 |
| Cg16 | 13 | 13.5 | 10 | 12 | 0 | 0 | 15.6 | 31.2 |
| Cg17 | 12 | 12.5 | 15.5 | 16 | 14.5 | 13 | 15.6 | 31.2 |
| Cg18 | 17.5 | 16.5 | 17.5 | 18 | 14.5 | 13 | 15.6 | 31.2 |
| Cg19 | 12.5 | 12.5 | 13.5 | 14.5 | 11.5 | 10 | 15.6 | 31.2 |
| Cg20 | 21 | 22 | 21 | 19 | 19 | 24.5 | 7.8 | 15.6 |
| Cg21 | 11 | 10.5 | 11.5 | 14 | 13 | 13.5 | 15.6 | 31.2 |
| Cg22 | 12.5 | 12 | 10.5 | 14 | 13 | 12.5 | 7.8 | 15.6 |
| Cg23 | 10.5 | 10.5 | 12.5 | 16 | 15.5 | 0 | 15.6 | 31.2 |
| Ave | 15.7 | 14.9 | 14 | 13.9 | 11.0 | 9.76 | 15.2 | 30.5 |
Antifungal Activity of Methanolic Extract of E. cephalotes against C. glabrata
| Con, mg/mL | 7.8 | 15.6 | 31.25 | 62.5 | 125 | 250 | MIC | MFC |
|---|---|---|---|---|---|---|---|---|
| Inhibition Zone, mm | ||||||||
| Cg1 | 24 | 24 | 24 | 29.5 | 18.5 | 24 | 7.8 | 15.6 |
| Cg2 | 26 | 22.5 | 21 | 18.5 | 16.5 | 16.5 | 31.2 | 62.5 |
| Cg3 | 12.5 | 15.5 | 13.5 | 13 | 12 | 11 | 15.6 | 31.2 |
| Cg4 | 14.5 | 14.5 | 15.5 | 15.5 | 15.5 | 15.5 | 7.8 | 15.6 |
| Cg5 | 13 | 14.5 | 0 | 0 | 0 | 0 | 7.8 | 15.6 |
| Cg6 | 24 | 21 | 14 | 15.5 | 15.5 | 0 | 7.8 | 15.6 |
| Cg7 | 11.5 | 12 | 11.5 | 12.5 | 12.5 | 0 | 15.6 | 31.2 |
| Cg8 | 19.5 | 18.5 | 19 | 13 | 13 | 13 | 7.8 | 15.6 |
| Cg9 | 24 | 24 | 24 | 12.5 | 14 | 14.5 | 7.8 | 15.6 |
| Cg10 | 12.5 | 16 | 15 | 15.5 | 14 | 11.5 | 7.8 | 15.6 |
| Cg11 | 12 | 17 | 17 | 9.5 | 0 | 0 | 7.8 | 15.6 |
| Cg12 | 14 | 14 | 0 | 0 | 0 | 0 | 15.6 | 31.2 |
| Cg13 | 15 | 13 | 13 | 10 | 0 | 0 | 7.8 | 15.6 |
| Cg14 | 16.5 | 15.5 | 14.5 | 13 | 12.5 | 12 | 15.6 | 31.2 |
| Cg15 | 15 | 13.5 | 13 | 12 | 10.5 | 10.5 | 15.6 | 31.2 |
| Cg16 | 12.5 | 13 | 12.5 | 0 | 0 | 0 | 7.8 | 15.6 |
| Cg17 | 13 | 14 | 15.5 | 14 | 13.5 | 13.5 | 15.6 | 31.2 |
| Cg18 | 19 | 18 | 18.5 | 12.5 | 11 | 10.5 | 15.6 | 31.2 |
| Cg19 | 12 | 12 | 14.5 | 12.5 | 12.5 | 10.5 | 15.6 | 31.2 |
| Cg20 | 22.5 | 20.5 | 20.5 | 16 | 6.5 | 13 | 15.6 | 31.2 |
| Cg21 | 12 | 11 | 11 | 15 | 15 | 14 | 7.8 | 15.6 |
| Cg22 | 17 | 16 | 16 | 15 | 14 | 11 | 7.8 | 15.6 |
| Cg23 | 11.5 | 13 | 14.5 | 16 | 15 | 0 | 15.6 | 31.2 |
| Ave | 16.2 | 16.2 | 14.6 | 12.6 | 10.5 | 8.73 | 12.2 | 24.4 |
Antifungal Activity of Aqoues Extract of E. cephalotes against C. glabrata
| Con, mg/mL | 7.8 | 15.6 | 31.25 | 62.5 | 125 | 250 | MIC | MFC |
|---|---|---|---|---|---|---|---|---|
| Inhibition Zone, mm | ||||||||
| Cg1 | 20.5 | 20.5 | 21 | 20 | 19.5 | 21 | < 7.8 | 7.8 |
| Cg2 | 26 | 21.5 | 21 | 20 | 15.5 | 20.5 | 15.6 | 31.2 |
| Cg3 | 11 | 13 | 13.5 | 16.5 | 14.5 | 15.5 | 15.6 | 31.2 |
| Cg4 | 17.5 | 17.5 | 16.5 | 14 | 16 | 21 | 15.6 | 31.2 |
| Cg5 | 16 | 19.5 | 20 | 15.5 | 4 | 12.5 | 15.6 | 31.2 |
| Cg6 | 21 | 18.5 | 15 | 4.5 | 13 | 19 | 15.6 | 31.2 |
| Cg7 | 18 | 15.5 | 15 | 17 | 18.5 | 15 | 7.8 | 15.6 |
| Cg8 | 14.5 | 14.5 | 15 | 13 | 12 | 10.5 | 15.6 | 31.2 |
| Cg9 | 19.5 | 20.5 | 27 | 23 | 16.5 | 21 | 15.6 | 31.2 |
| Cg10 | 22.5 | 19 | 17 | 17 | 17 | 13 | 15.6 | 31.2 |
| Cg11 | 12.5 | 12 | 10.5 | 13 | 12.5 | 4 | 31.2 | 62.5 |
| Cg12 | 16 | 16 | 12.5 | 10.5 | 3.5 | 11 | 31.2 | 62.5 |
| Cg13 | 18 | 18 | 15.5 | 16 | 17.5 | 15.5 | 31.2 | 62.5 |
| Cg14 | 18 | 11 | 11 | 15.5 | 14.5 | 15 | 31.2 | 62.5 |
| Cg15 | 14.5 | 17 | 7.5 | 19 | 21 | 16 | 31.2 | 62.5 |
| Cg16 | 10.5 | 12 | 6 | 13 | 15 | 16 | 31.2 | 62.5 |
| Cg17 | 15.5 | 16.5 | 17 | 15.5 | 16 | 15.5 | 31.2 | 62.5 |
| Cg18 | 0 | 0 | 0 | 15 | 15.5 | 15 | 31.2 | 62.5 |
| Cg19 | 19 | 16 | 18 | 15 | 15 | 4.5 | 15.6 | 31.2 |
| Cg20 | 16.5 | 16.5 | 14.5 | 14 | 15 | 20 | 15.6 | 31.2 |
| Cg21 | 21.5 | 16.5 | 16 | 10 | 12.5 | 12 | 15.6 | 31.2 |
| Cg22 | 11.5 | 6.5 | 12 | 19 | 14 | 13.5 | 7.8 | 15.6 |
| Cg23 | 0 | 0 | 13.5 | 14.5 | 13.5 | 4.5 | 15.6 | 31.2 |
| Ave | 18 | 17.8 | 17.8 | 16.6 | 15.1 | 15.3 | 20.5 | 39.7 |
Thus in C. parapsilosis isolate the antifungal activity that of aqueous extract than the ethanolic and methanolic extracts is better. Ethanolic, methanolic and aqueous extracts of trehala manna not show any antifungal activity. In this study, fluconazole, as a positive control showed antifungal activity with inhibition zone of 0 till 75 mm. Many of Candida species showed similar sensitivity to fluconazole and E. cephalotes. The MIC of the plant extracts varying between 7.8 - 250 mg/mL were tested.
Our results revealed a strong antifungal activity of methanolic extracts of E. cephalotes with MIC values varying from < 7.8 to 7.8 mg/mL and moderate antifungal activity of ethanolic extract with MIC values 15.6 mg/mL and less activity of aqueous extract with MIC values 31.2 mg/mL. C. albicans and C.glabrata were the most sensitive strains to the tested extracts. The lowest MICs were obtained with methanolic and aqueous extracts for C. albicans (< 7.8 mg/mL) and the highest MICs were obtained for aqueous extracts on C. tropicalis (31.2 mg/mL).
5. Discussion
According this research, E. cephalotes extract had strong antifungal activity and the development of new therapeutic agents, as they inhibited the growth of Candida. Subsequently determination of E. cephalotes fractionations is nessessary to identify antifungal compounds. As a result the plant E. cephalotes can play an important role in the treatment of infectious diseases, particularly vulvovaginal candidiasis. Some researchers were reported that the genus of Echinops are consist of flavonoids, alkaloids, saponins, phytosterols, polyphenols, carotenoids, sesquiterpene lactones/alcohols, lignans, acetylenic and thiophene compounds and essential oils. Flavonoids have two main roles in plants, in leaves, protecting it from fungal pathogens and UV-B radiation [23]. Although the findings of this research can be due to the existence of compounds contained in the plant E. cephalotes, including cellulosic materials, starch and sugar trehalose. E. cephalotes plant with there sugar compounds include trehalose from ancient times until now, attention [24]. Based on obtained results in current study, it seemed that E. cephalotes have useful medical components that identification it, is valuable. Darwish and Aburjai observed effectiveness of methanol extract of E. polyceras against C. albicans with an MIC value of 6.3 mg/mL [13]. Abd-Ellatif et al. found positive results for methanol extract of E. spinosissimus with zone of inhibition 13 mm against Candida species [25]. Machocho showed strong antifungal activity of crude extract of E. hispidus and the other effective compounds were polyacetylene thiophenes which gave an average inhibition of 23 mm [26]. Fokialakis et al. studied on antifungal activity of thiophenes from the dichloromethane extract of the radix of E. ritro that according to the result compounds 5-(3-buten-1-ynyl)-2, 2-bithiophene; R-terthienyl and 2-[pent-1, 3-diynyl]-5-[4-hydroxybut-1-ynyl] thiophene had significant activity against Colletotrichum species and Fusarium [27]. Muithya and Machocho reported that copmopund of polyacetylene thiophenes; 2-Octyl-5-(3, 4-dihydroxybut-1-enyl) thiophene of E. hispidus showed no antifungal activity but compounds of [4-[5-(penta-1, 3-dieynyl) thien-2-yl] but-3-ynyl alcohol and 2-(penta-1,3-diynyl)-5-(3, 4 dihydroxybut-1-ynyl) thiophene of E. hispidus in the family of Asteraceae exhibited very strong antifungal activities against C. neofrmance [28]. Hymete et al. reported the flower extract of E. ellenbeckii showed inhibitory effect against C. albicans and leaf and stem extracts of E. longisetus inhibited the growth of S. aureus. The aureus (pl. aurei) was a gold coin of ancient Rome valued at 25 silver denarii. The aureus was regularly issued from the 1st century BC to the beginning of the 4th century AD, when it was replaced by the solidus in a dose dependent manner [29]. Toroglu et al. reported the ethyl acetat extracts of leaves of E. vicosus showed antifungal activity against M. pusilus with 11 mm inhibition zone and the ethanol extract of flower of E. microcephalus presented antifungal activity against M. pusilus with 7 mm inhibition zone [23]. Data obtained from other studies demonstrated positive results for these plants as well. Although these result is not exactly accordance with ours. There may be many factors such as the different species of Echinops. Fluconazole, which is commonly used for treatment of various fungal infections, considered as a fungistatic agent and there are reports of emerging resistance among clinical isolates of C. albicans [30]. In this context, natural products have been used historically and continue to be the focus of researches on antifungal agents. The main source is to be found in plants. Many plant extracts have biological activity both in vitro and in vivo [31]. Difference of susceptibility to fluconazole and E. cephalotes may be due to the indiscriminate use of fluconazol in patients with vulvovaginit candidiasis. This research is the first study on antifungal activity of the E. cephalotes against Candida spp. isolated from patients with vulvovaginal candidiasis. Hence, this plant might be a promising material for controlling Candida spp. and may used further as medicinal plant against Candida spp. Also the results from the present study have reported the scientific basis for traditional uses of the genus Echinops in the treatment of some illness. Since the identifying of active phytochemical compounds has conducted by researchers, more investigation should be done in vitro and in vivo for safety. After that stage, it can be produced commercially.
Acknowledgements
References
-
1.
Pappas PG, Kauffman CA, Andes D, Benjamin DJ, Calandra TF, Edwards JJ, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48(5):503-35. [PubMed ID: 19191635]. https://doi.org/10.1086/596757.
-
2.
Costa M, Passos XS, Miranda AT, de Araujo RS, Paula CR, Silva Mdo R. Correlation of in vitro itraconazole and fluconazole susceptibility with clinical outcome for patients with vulvovaginal candidiasis. Mycopathologia. 2004;157(1):43-7. [PubMed ID: 15008344].
-
3.
Pakshir K, Zomorodian K, Karamitalab M, Jafari M, Taraz H, Ebrahimi H. Phospholipase, esterase and hemolytic activities of Candida spp. isolated from onychomycosis and oral lichen planus lesions. J Mycol Med. 2013;23(2):113-8. [PubMed ID: 23706304]. https://doi.org/10.1016/j.mycmed.2013.04.007.
-
4.
Randhawa MA. The effect of dimethyl sulfoxide (DMSO) on the growth of dermatophytes. Nihon Ishinkin Gakkai Zasshi. 2006;47(4):313-8. [PubMed ID: 17086165].
-
5.
Lopes Consolaro ME, Aline Albertoni T, Shizue Yoshida C, Mazucheli J, Peralta RM, Estivalet Svidzinski TI. Correlation of Candida species and symptoms among patients with vulvovaginal candidiasis in Maringa, Parana, Brazil. Rev Iberoam Micol. 2004;21(4):202-5. [PubMed ID: 15709802].
-
6.
Richter SS, Galask RP, Messer SA, Hollis RJ, Diekema DJ, Pfaller MA. Antifungal susceptibilities of Candida species causing vulvovaginitis and epidemiology of recurrent cases. J Clin Microbiol. 2005;43(5):2155-62. [PubMed ID: 15872235]. https://doi.org/10.1128/JCM.43.5.2155-2162.2005.
-
7.
Calixto JB. Twenty-five years of research on medicinal plants in Latin America: a personal view. J Ethnopharmacol. 2005;100(1-2):131-4. [PubMed ID: 16006081]. https://doi.org/10.1016/j.jep.2005.06.004.
-
8.
de Oliveira JR, de Castro VC, das Gracas Figueiredo Vilela P, Camargo SE, Carvalho CA, Jorge AO, et al. Cytotoxicity of Brazilian plant extracts against oral microorganisms of interest to dentistry. BMC Complement Altern Med. 2013;13:208. [PubMed ID: 23945270]. https://doi.org/10.1186/1472-6882-13-208.
-
9.
Pfaller MA. Antifungal drug resistance: mechanisms, epidemiology, and consequences for treatment. Am J Med. 2012;125(1 Suppl):S3-13. [PubMed ID: 22196207]. https://doi.org/10.1016/j.amjmed.2011.11.001.
-
10.
Sadeghi-Nejad B, Shiravi F, Ghanbari S, Alinejadi M. Antifungal activity of Satureja khuzestanica (Jamzad) leaves extracts. Jundishapur J Microbiol. 2010;3(1):36-40.
-
11.
Gültekİn L, Podlussány A. Two new species of Larinus from Iran (Coleoptera: Curculionidae: Lixinae). Acta Entomol Mus Nat Pragae. 2012;52:245-58.
-
12.
Nasirzadeh AAR, Javidtash I, Riasat M. Identification of Echinops species and study on some biological characteristics of larinus vulpes ouv. As manna producer in Fars province. Aromat Plants Res. 2005;21(3):335-46.
-
13.
Darwish RM, Aburjai TA. Antimicrobial activity of some medicinal plants against different Candida species. Jordan J Pharm Sci. 2011;4(1).
-
14.
Odds FC, Bernaerts R. CHROMagar Candida, a new differential isolation medium for presumptive identification of clinically important Candida species. J Clin Microbiol. 1994;32(8):1923-9. [PubMed ID: 7989544].
-
15.
Lee JA, Chee HY. In Vitro Antifungal Activity of Equol against Candida albicans. Mycobiology. 2010;38(4):328-30. [PubMed ID: 23956675]. https://doi.org/10.4489/MYCO.2010.38.4.328.
-
16.
Diba K, Shoar MG, Shabatkhori M, Khorshivand Z. Anti fungal activity of alcoholic extract of Peganum harmala seeds [in Persian]. Urmia Med J. 2011;4(1):271-7.
-
17.
Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically;. 29. 2009. p. 1-12.
-
18.
Reference method for broth dilution antifungal susceptibility testing of yeasts. 28. 3 ed. USA: CLSI; 1992. p. 1-25.
-
19.
Johann S, Pizzolatti MG, Donnici CL, Resende MA. Antifungal properties of plants used in Brazilian traditional medicine against clinically relevant fungal pathogens. Brazil J Microb. 2007;38(4):632-7. https://doi.org/10.1590/s1517-83822007000400010.
-
20.
Espinel-Ingroff A, Fothergill A, Peter J, Rinaldi MG, Walsh TJ. Testing conditions for determination of minimum fungicidal concentrations of new and established antifungal agents for Aspergillus spp.: NCCLS collaborative study. J Clin Microbiol. 2002;40(9):3204-8. [PubMed ID: 12202554].
-
21.
Silveira CP, Torres-Rodriguez JM, Alvarado-Ramirez E, Murciano-Gonzalo F, Dolande M, Panizo M, et al. MICs and minimum fungicidal concentrations of amphotericin B, itraconazole, posaconazole and terbinafine in Sporothrix schenckii. J Med Microbiol. 2009;58(Pt 12):1607-10. [PubMed ID: 19679687]. https://doi.org/10.1099/jmm.0.007609-0.
-
22.
Mathur A, Singh R, Yousuf S, Bhardwaj A, Verma SK, Babu P, et al. Antifungal activity of some plant extracts against clinical pathogens. Adv Appl Sci Res. 2011;2(2):260-4.
-
23.
Toroğlu S, Keskin D, Vural C, Kertmen M, Çenet M. Comparison of antimicrobial activity of Echinops viscosus subsp. Bithynicus and E. microcephalus leaves and flowers extracts from Turkey. Int J Agric Biol. 2012;14(4):637-40.
-
24.
Takavar S, Mohammadi M. Producer's factors and mechanisms of manna in iran [in Persian]. J Med Plants. 2008;7(4):28-37.
-
25.
Abd-Ellatif S, Abdel Rahman SM, Deraz SF. Promising antifungal effect of some folkloric medicinal plants collected from El-Hammam habitat, Egypt against dangerous pathogenic and toxinogenic fungi. ARPN J Agric Biol Sci. 2011;6(9):25-32.
-
26.
Machocho AK. Antimicrobial activity and phytochemical studies of some selected medicinal kenyan plants. Proceeding of the 14th NAPRECA Symposium and AAMPS Ethnoveterinary Medicine Symposium. Nairobi: International Center for Insect Physiology and Ecology; 2011.
-
27.
Fokialakis N, Cantrell CL, Duke SO, Skaltsounis AL, Wedge DE. Antifungal activity of thiophenes from Echinops ritro. J Agric Food Chem. 2006;54(5):1651-5. [PubMed ID: 16506815]. https://doi.org/10.1021/jf052702j.
-
28.
Muithya JN. Phytochemical and in Vitro Anti-Microbial Screening of Echinops Hispidus Fresen. and Grewia Similis K. Schum. Nairobi: Kenyatta University; 2010.
-
29.
Hymete A, Iversen TH, Rohloff J, Erko B. Screening of Echinops ellenbeckii and Echinops longisetus for biological activities and chemical constituents. Phytomedicine. 2005;12(9):675-9. [PubMed ID: 16194056]. https://doi.org/10.1016/j.phymed.2004.01.013.
-
30.
Agarwal V, Lal P, Pruthi V. Effect of plant oils on Candida albicans. J Microbio, Immunol Infect. 2010;43(5):447-51.
-
31.
Sousa ZL, de Oliveira FF, da Conceicao AO, Silva LA, Rossi MH, Santos Jda S, et al. Biological activities of extracts from Chenopodium ambrosioides Lineu and Kielmeyera neglecta Saddi. Ann Clin Microbiol Antimicrob. 2012;11:20. [PubMed ID: 22839690]. https://doi.org/10.1186/1476-0711-11-20.