Abstract
Background:
Inappropriate prescriptions of antibiotics in children is common in most parts of the world.Objectives:
This study was performed to evaluate antibiotic prescription patterns and the rational use of antibiotics in hospitalized children in a tertiary referral hospital in Kerman, Iran.Methods:
This prospective cross-sectional study evaluated prescription patterns of antibiotics for hospitalized children under 12 years old for six months from October 1, 2017, to March 31, 2018. Demographic information, including age, sex, most commonly-prescribed drug classes, name of antibiotic, duration and route of administration, diagnosis, and results of microbial culture and duration of hospitalization were recorded.Results:
Most antibiotics were administered by parenteral route. The major infections for which antibiotics were prescribed were febrile seizures (34.4%), upper respiratory infections (25.5%), pyrexia with unknown origin (17.6%), and urinary tract infections (14.8%). Ceftriaxone, clindamycin, and vancomycin were the most frequently prescribed antibiotics. The selection of 84% of antibiotics was on an empirical basis. Ampicillin (83.9%) and clindamycin (81.0%) showed the highest resistance rate, followed by ceftriaxone (33.0%) and vancomycin (32.0%).Conclusions:
Our results showed inappropriate use of antibiotics in hospitalized children. A relatively high resistance rate to prescribed antibiotics for children indicates that the selection of high-cost antibiotics, including ceftriaxone, vancomycin, and meropenem should be based on antimicrobial sensitivity tests.Keywords
Antibiotic Pediatrics Prescription Hospitalized Children Iran
1. Background
Studies on drug utilization are useful tools to assess the rational prescriptions by health care professionals for both outpatients and hospitalized patients. The high prevalence of antimicrobial use for children, particularly systemic antibiotic agents, is common in both developed and developing countries (1-4). Previous studies indicate that antibiotics are prescribed for approximately 50% of (74.8% in Sierra Leon and 49.5% in Nigeria) hospitalized infants and children in under developing countries and 42.9% in Germany, a developed country (4-6).
Inappropriate use of antibiotics is reported in most parts of the world, which promotes the emergence and expansion of antibiotic-resistant organisms in both adults and children (4, 7, 8). Infections due to antimicrobial-resistance organisms are associated with a significant increase in patient morbidity and mortality and high hospitalization charges (9, 10). Also, antibiotic prescribing leads to the emergence of adverse drug reactions, including allergic reactions and pseudomembranous enterocolitis superinfection due to Clostridium difficile (10, 11). Katherine et al. (2016) reported inappropriate oral antibiotic prescriptions for 30% of US ambulatory care visits outpatients in the United States (12). In most studies, acute respiratory conditions, including pneumonia, otitis media, pharyngitis, bronchitis, and influenza, were the prevalent reasons for antibiotic consumption in children (4, 6, 13). United States Centers for Disease Control and Prevention (CDC) reported that bacterial infections due to antibiotic-resistant microorganisms affect two million people and are responsible for 23,000 deaths annually in the USA (12).
Penicillins, cephalosporins, Macrolides, aminoglycosides, sulfonamides, and fluoroquinolones are the most common antibacterial prescribed for children; however, there is a wide variation between the pattern of antibiotic prescription for children in different countries. Nevertheless, ceftriaxone, amoxicillin, amoxicillin-clavulanic acid, penicillin, V, and clarithromycin were the most frequently prescribed antibiotics (6, 13).
2. Objectives
Since there was no current information about the pattern of antibiotic prescription for hospitalized children in Iran, the objective of this study was to assess the current patterns of antibiotic prescribing among pediatric patients under 12 years admitted to a tertiary referral teaching hospital in Kerman, Iran.
3. Methods
This study was a prospective cross-sectional that evaluate the prescription pattern of antibiotics for hospitalized children under 12 years old during a 6-month period from October 1, 2017, to March 31, 2018, at the pediatric department of 350-bed Afzalipour teaching hospital at Kerman, southeast of Iran. The Afzalipour hospital is the main reference public hospital in Kerman Province and provides healthcare services for inpatients and on referral from other medical centers in Kerman province, irrespective of the patient's socioeconomic characteristics. Kerman province is situated 1,000 km from Tehran in the south of Iran. The protocol was approved by the Ethics Committee of Kerman University of Medical Sciences (Ethics code: IR.KMU.AH.REC.1396.2208).
The inclusion criteria were all hospitalized children with a known diagnosis of infectious disease in the pediatric infectious ward aged one month to 12 years who received at least one antibiotic during the hospitalization period. Neonates aged 0 to 1 month and children hospitalized in other wards of pediatric departments were excluded. Demographic information, including age, sex, name of antibiotic, duration and route of administration, diagnosis, results of microbial culture, and duration of hospitalization, were recorded. Patients specimens were sent for culture and sensitivity testing, the organisms isolated, and their antibiotic sensitivity patterns were determined by disc diffusion methods. The most commonly prescribed drug classes and the percentage of drugs prescribed were calculated.
3.1. Statistical Analysis
The data were analyzed using SPSS version 22 (SPSS Inc., Chicago) statistical program software. Results are presented as percentages, means, and standard deviations. Paired t-test was used to determine the significant differences in the average time of hospitalization within each group. Moreover, P ≤ 0.05 was considered statistically significant.
4. Results
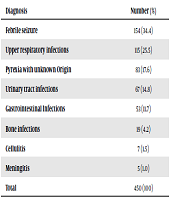
In this study, the data prescription of 450 children (mean age: 2.8 ± 2.3 years, range: one month to 12 years) under 12 years old were studied in Afzalipour hospital pediatric ward. Table 1 shows the patients' characteristics. Our results showed that 34% of children were under one year old, and 53% of patients were male. There was no significant difference in the average time of hospitalization (5.1 ± 2.1 days in febrile vs. 5.2 ± 3.1 days in non-febrile children, P = 0.49). Table 2 shows the diagnostic pattern of hospitalized children. Febrile seizures (34.4%), upper respiratory tract infections (25.5%), and pyrexia with unknown origin (17.6%) were the most frequent causes of antibiotic prescription.
Most of antibiotics were administered by intravenous (IV) infusion (67.6%) followed by the combination of oral and IV (24.9%) and oral administration (7.5%).
Demographic Characteristics of Hospitalized Children Aged One Month to 12 Years in Afzalipour Hospital Pediatric Ward, Kerman, Iran
| Parameters | Number (%) |
|---|---|
| Age | |
| 1 month-1 year | 155 (34) |
| 1-2 years | 105 (23) |
| 2-3 years | 86 (19) |
| 3-4 yeas | 64 (14) |
| ≥ 4 years | 40 (8) |
| Mean ± SD | 2.8 ± 2.3 |
| Sex | |
| Females | 209 (47) |
| Males | 241 (53) |
Diagnosis Pattern of Hospitalized Children Aged One Month to 12 Years in Afzalipour Hospital Pediatric Ward, Kerman, Iran
| Diagnosis | Number (%) |
|---|---|
| Febrile seizure | 154 (34.4) |
| Upper respiratory infections | 115 (25.5) |
| Pyrexia with unknown Origin | 83 (17.6) |
| Urinary tract infections | 67 (14.8) |
| Gastrointestinal Infections | 53 (11.7) |
| Bone infections | 19 (4.2) |
| Cellulitis | 7 (1.5) |
| Meningitis | 5 (1.0) |
| Total | 450 (100) |
Blood/urine specimens were obtained from children and routine microbial cultures (blood agar and MacConkey's agar) were performed for the identification of pathogens and susceptibility testing by disk diffusion test. Since the microbial culture was performed on patients’ urine and blood samples, so the microbial isolation and antibiotic resistance for patients with bone infection and cellulitis was not determined. Of 450 specimen received by the microbiological laboratory, only 72 (16%) cases showed positive culture. All 450 hospitalized patients received antibiotics, and a total of 378 (84%) of hospitalized children received antibiotics on an empirical basis. Escherichia coli, streptococcus β-hemolytic, viridans streptococci, Shigella, and Staphylococcus aureus species were the common organisms isolated.
Our results showed that the selection of antibiotics in 84% of hospitalized children was chosen on an empirical basis. Beta-lactam antibiotics (86.4%) were the most frequently prescribed antibiotics followed by macrolides (13.3%) and vancomycin (3.3%) (Table 3).
Distribution of the Prescribed Antibacterial Drug Category in Hospitalized Children in Afzalipour Hospital, Kerman, Iran.
| Drug Category | Number (%)a |
|---|---|
| Beta-lactams | 389 (86.4) |
| Macrolides | 60 (13.3) |
| Clindamycin | 51 (11.3) |
| Vancomycin | 15 (3.3) |
| Metronidazole | 11 (2.4) |
| Aminoglycosides | 9 (2.0) |
| Quinolones | 6 (1.4) |
| Antivirals | 7 (2.0) |
| Others | 38 (8.4) |
Ceftriaxone (82.0 %), a 3rd generation cephalosporin, was the most commonly prescribed antibiotic, followed by clindamycin (11.3%), vancomycin (3.3%), cefotaxime + ampicillin (3.1%), and meropenem (1.1%). A combination of antibiotics was used in 51 (11.3%) of children, and Ceftriaxone (8.2%) was the frequent antibiotic in combination therapy (Table 4). Cefazolin, cefepime, ceftazidime, cefixime, azithromycin, erythromycin, metronidazole, and ciprofloxacin were other antibiotics prescribed for a very few patients (Table 4).
Most Commonly Used Antibacterials/Antivirals in Hospitalized Children Aged One Month to 12 Years in Afzalipour Hospital Pediatric Ward, Kerman, Irana
| Drug name | Number (%) |
|---|---|
| Ceftriaxone | 369 (82.0) |
| Clindamycin | 51 (11.3) |
| Vancomycin | 15 (3.3) |
| Cefotaxime + ampicillin | 14 (3.1) |
| Ceftriaxone + vancomycin | 11 (2.4) |
| Ceftriaxone + clindamycin | 7 (1.6) |
| Ceftriaxone + metronidazole | 4 (0.9) |
| Ceftriaxone + amikacin | 9 (2.0) |
| Ceftriaxone + azithromycin | 4 (0.9) |
| Ceftriaxone + acyclovir | 2 (0.4) |
The highest antimicrobial sensitivity was observed to meropenem (92%), followed by cefotaxime (73.3%), ceftazidime (68.8%), vancomycin (68.0%), and ceftriaxone (67.0%), and the highest resistance rate was observed to ampicillin (83.9%) and clindamycin (81.0%) (Table 5). Staphylococcus aureus species showed the highest rate of bacterial resistance to prescribed antibiotics, and streptococcus β-hemolytic was sensitive to all prescribed antibiotics.
Sensitivity and Resistance Rate to Commonly Used Antibiotics in Hospitalized Children Aged One Month to 12 years in Afzalipour Hospital, Kerman, Iran
| Antibiotic | Culture Analysis | |
|---|---|---|
| Sensitive (%) | Resistant (%) | |
| Meropenem | 92.0 | 8.0 |
| Cefotaxime | 73.3 | 26.7 |
| Ceftazidime | 68.8 | 21.2 |
| Vancomycin | 68.0 | 32.0 |
| Ceftriaxone | 67.0 | 33.0 |
| Cefixime | 61.3 | 38.7 |
| Cefazolin | 55.0 | 45.0 |
| Ciprofloxacin | 53.8 | 46.2 |
| Clindamycin | 19.0 | 81.0 |
| Ampicillin | 16.1 | 83.9 |
5. Discussion
The present study assessed the use of different antibiotic classes in the treatment of infections in hospitalized children under 12 years old. In our study, the intravenous infusion route was the most common form of antibiotic administration followed by the combination of parenteral and oral route for pediatric patients, which is similar to other studies in Ethiopia and India (13, 14), but it is higher than similar study in Nepal (15). Our results showed that febrile convulsions (34.4%) were the most frequent indication for antibiotic prescription, followed by upper respiratory tract infections (25.5%) and pyrexia with unknown origin (17.6%). In some other studies, pneumonia is the leading cause of hospitalization in children (13, 14, 16). Acute otitis media and upper respiratory tract infection were the main clinical findings in hospitalized children in Germany, and fever was the most frequent cause of children’s hospitalization in Saudi Arabia (6, 17). Acute gastroenteritis and respiratory illnesses are the major causes of morbidity and mortality in children under five years of age, for which inappropriate antibiotic utilization for the treatment of cough/cold and/or diarrhea in pediatric patients is the most common (18). In most cases, no antibiotic therapy is needed for the treatment of acute diarrhea in children, and rehydration is the key treatment (19).
This study showed that 3rd generation cephalosporins were the most frequently prescribed antibiotics, followed by macrolides, vancomycin, and metronidazole. Ceftriaxone was the most commonly prescribed antibiotic, followed by clindamycin and vancomycin. There is variability in antibiotic prescription for hospitalized children and typically the prescription of a higher proportion of broad-spectrum antibiotics. Although cephalosporins, macrolides, vancomycin, and metronidazole are the most frequently prescribed antibiotic classes for children, their pattern of administration is varied in different countries (4, 6, 17). Cephalosporins have been the most frequently prescribed antibiotic classes in hospitalized children in many countries including Canada (20), Saudi Arabia (17), India (14), Nepal (16), and United States (2). Macrolides were the most frequently prescribed antibiotic classes for children in Germany (6); however, penicillins were the most common antibiotics in Brazil (7) and Greece (21).
Consistent with this study, Ceftriaxone 369 (82.0%), clindamycin 51 (11.3%), and vancomycin 15 (3.3%) were the three most commonly used antibiotics in hospitalized children. Meropenem, a carbapenem antibiotic, was prescribed in 1.1% of children and 3.1% of patients received the combination of cefotaxime and ampicillin. Cefazoline, 1st generation cephalosporin, was the most frequently antibiotic used in Canada (20) and United States (2). In our study ampicillin was the most frequently prescribed penicillin, which was used in combination with cefotaxime, a 3rd generation cephalosporin, in only 3.1% of the patients. However, in similar studies, amoxicillin was the most frequently prescribed antibiotics in Brazil (7), Greece (21), India (14). Gentamicin has been used as the first choice for hospitalized infants in NICU patients in the United States (2) and pediatric patients in Nigeria (4).
The 3rd generation cephalosporins (ceftriaxone and cefotaxime) and carbapenems (meropenem) are among expensive drugs and should usually be reserved for treatment of serious infections caused by organisms resistant to other antibiotics, including penicillin-resistant pneumococci (PRSP strains). Also, vancomycin is used for the treatment of drug-resistant gram-positive organisms, including methicillin-resistant staphylococci (MRSA), and penicillin-resistant pneumococci (PRSP) that is used in combination with a third-generation cephalosporin. Since in this study, most antibiotics (84%) are prescribed on an empirical basis, the high rate prescription of these antibiotics could be an indication of inappropriate use of antibiotics. In this regard, antibiotic restriction policies should be applied for the promotion of the rational use of antibiotics, thereby resulting in a significant reduction of antibiotic use and hospitalization costs (22). Vancomycin was among the most commonly prescribed antibiotics for hospitalized children in Canada (20) and NICU patients in the United States (2). Inappropriate use of fluoroquinolones for hospitalized pediatric patients is reported in Brazil (7), Saudi Arabia (17) and India (14).
In our studies, the hospitalized children received antibiotics for more than five days (5.1 d in febrile vs 5.2 d in non-febrile patients). It is reported that empirical antibiotic therapy for more than five days increases the risk of necrotizing enterocolitis or death in extremely low birth weight infants (23). Also, antibiotic therapy increase the risk of Clostridium difficile infection, ranging from severe diarrhea, pseudomembranous colitis, toxic megacolon, bowel perforation, and death in hospitalized children (24). Qureshi et al. (2013) reported that hospitalization of patients with transient ischemic attacks for more than two days has been associated with 2-5 times higher hospitalization costs (25). Fine et al. (2000) reported a considerable reduction in expenses after 1-day reduction in hospitalization period for patients with community-acquired pneumonia (26).
In this study, the selection of antibiotics was based on the antimicrobial sensitivity test in 84 % of patients who received antibiotics on an empirical basis and only in 16% of children. It is noteworthy that most of the hospitalized children were referred from other pediatric departments in Kerman Province and were received antibiotics before admission. Similar studies in different countries, including Singapore (27), Tennessee state in the United States (28), Tanzania (29), Nepal (15) , India (14), and Ethiopia (13) reported the empirical antibiotic therapy for hospitalized children. Wang et al. (2019) reported inappropriate empirical antibiotic choice in children hospitalized for atopic dermatitis (30)
Antimicrobial sensitivity test showed that ampicillin was associated with the highest rate of resistance to prescribed antibiotics (83.9%), followed by clindamycin (81.0%). Also, the resistance rate to other prescribed antibiotics was relatively high, ranging from 8.0% to meropenem, 26.7% to cefotaxime, 32.0% to vancomycin, and 33.0% to ceftriaxone, which is higher than the resistance rate to ampicillin (74.2%), ceftriaxone (7.5%), and co-trimoxazole (61.3%) in Turkish children with urinary tract infections, and 5% resistance to cefotaxime in the United States (31). Similar studies on high resistance rate of commonly used antibiotics in Iranian hospitalized children have been reported previously (32-34). Ghorashi et al. (2011) reported that more than 95% of isolated pathogens from children’s urinary tract infections were resistant to ampicillin and resistance rate to cefotaxime and ceftriaxone was 27.6% and 22.4%, respectively (32). In agreement with our results, Mehrgan et al. (2008)) reported that extended-spectrum β-lactamase-producing (ESBL) Escherichia coli (E. coli) were highly susceptible to imipenem (100%), amikacin (91.1%), and piperacillin/tazobactam (85.2%) (33). Contrary to our results, Rezai et al. (2015) reported a very high resistance rate of ESBL-producing E. coli isolates to cefixime (99%), colistin (82%), and ciprofloxacin (76%) among pediatrics in the North of Iran (34). Also, Kocak et al. (2016) reported a high rate of antimicrobial resistance among ESBL-producing E. coli to the 3rd generation cephalosporins in Turkish children hospitalized for urinary tract infection (35). Previous studies demonstrate that hospitalization is the major risk factor for the emergence and expansion of methicillin-resistant staphylococcus aureus (MRSA) in the community (36).
However, inadequate antibiotic use for the treatment of bacterial infections is an important factor in the emergence and expansion of antibiotic-resistant bacterial species. Group discussion of treatment guidelines and workshops for rational antibiotic prescription can improve the use of antimicrobials in hospitalized children.
Inappropriate use of antibiotics is a common problem in medicine. Also, the prescription of unapproved or off-label antibiotics in hospitalized children is common and off-label for dose is the most prevalent category (37). So, accurate prescription of antibiotics must be observatory for not only for high-cost antibiotics but also for all antibiotics. The increased use of newer antibiotics is associated with an overall rise in healthcare costs as well as the faster development of bacterial resistance and the emergence of antibiotic-resistant microbial species throughout the world (9). So healthcare professionals should apply strategies for monitoring and control of antibiotic use to reduce both antibiotic resistance and adverse events. Strategies for reduction of prescription of wide-spectrum antibiotics, including 2nd and 3rd generation cephalosporins, carbapenems, and quinolones, can be useful for the prevention of antimicrobial resistance (38).
5.1. Conclusions
Our results indicate inappropriate use of antibiotics in hospitalized children. Ceftriaxone, an expensive 3rd generation cephalosporin, was the most frequently prescribed antibiotic. Results showed a high rate of antimicrobial resistance to the most commonly prescribed antibiotics, and moderate resistance to the more expensive antibiotics was observed in hospitalized children. Although prescription of low-cost antibiotics should be encouraged; however, the prescription of high-cost antibiotics, including ceftriaxone, vancomycin, and meropenem, should be allowed merely on the basis of antimicrobial sensitivity tests. So rational and appropriate use of antibiotics by health professionals, through the selection of antibiotics using antimicrobial sensitivity test, as well as an appropriate dose and duration, is of vital importance.
Acknowledgements
References
-
1.
Gadzhanova S, Roughead E. Prescribed antibiotic use in Australian children aged 0-12 years. Aust Fam Physician. 2016;45(3):134.
-
2.
Grohskopf LA, Huskins WC, Sinkowitz-Cochran RL, Levine GL, Goldmann DA, Jarvis WR, et al. Use of antimicrobial agents in United States neonatal and pediatric intensive care patients. Pediatr Infect Dis J. 2005;24(9):766-73. [PubMed ID: 16148841]. https://doi.org/10.1097/01.inf.0000178064.55193.1c.
-
3.
Mathur P. Antimicrobial consumption in hospitals of developing nations: When will the Gap Bridge between infection rates and prescription patterns? Indian J Med Microbiol. 2016;34(1):3.
-
4.
Umar LW, Isah A, Musa S, Umar B. Prescribing pattern and antibiotic use for hospitalized children in a Northern Nigerian Teaching Hospital. Ann Afr Med. 2018;17(1):26-32. [PubMed ID: 29363633]. [PubMed Central ID: PMC5810090]. https://doi.org/10.4103/aam.aam_44_17.
-
5.
Cole CP, James PB, Kargbo AT. An evaluation of the prescribing patterns for under-five patients at a Tertiary Paediatric Hospital in Sierra Leone. J Basic Clin Pharm. 2015;6(4):109-14. [PubMed ID: 26692736]. [PubMed Central ID: PMC4660481]. https://doi.org/10.4103/0976-0105.168051.
-
6.
Schindler C, Krappweis J, Morgenstern I, Kirch W. Prescriptions of systemic antibiotics for children in Germany aged between 0 and 6 years. Pharmacoepidemiol Drug Saf. 2003;12(2):113-20. [PubMed ID: 12642974]. https://doi.org/10.1002/pds.786.
-
7.
Del Fiol Fde S, Lopes LC, Barberato-Filho S, Motta Cde C. Evaluation of the prescription and use of antibiotics in Brazilian children. Braz J Infect Dis. 2013;17(3):332-7. [PubMed ID: 23607920]. https://doi.org/10.1016/j.bjid.2012.10.025.
-
8.
Quan-Cheng K, Jian-Guo W, Xiang-Hua L, Zhen-Zhen L. Inappropriate use of antibiotics in children in China. Lancet. 2016;387(10025):1273-4. https://doi.org/10.1016/s0140-6736(16)30019-8.
-
9.
Cosgrove SE. The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clin Infect Dis. 2006;42 Suppl 2:S82-9. [PubMed ID: 16355321]. https://doi.org/10.1086/499406.
-
10.
Linder JA. Editorial commentary: antibiotics for treatment of acute respiratory tract infections: decreasing benefit, increasing risk, and the irrelevance of antimicrobial resistance. Clin Infect Dis. 2008;47(6):744-6. [PubMed ID: 18694343]. https://doi.org/10.1086/591149.
-
11.
Lo Vecchio A, Zacur GM. Clostridium difficile infection: an update on epidemiology, risk factors, and therapeutic options. Curr Opin Gastroenterol. 2012;28(1):1-9. [PubMed ID: 22134217]. https://doi.org/10.1097/MOG.0b013e32834bc9a9.
-
12.
Fleming-Dutra KE, Hersh AL, Shapiro DJ, Bartoces M, Enns EA, File TJ, et al. Prevalence of Inappropriate Antibiotic Prescriptions Among US Ambulatory Care Visits, 2010-2011. JAMA. 2016;315(17):1864-73. [PubMed ID: 27139059]. https://doi.org/10.1001/jama.2016.4151.
-
13.
Feleke M, Yenet W, Lenjisa J. Prescribing pattern of antibiotics in pediatric wards of Bishoftu Hospital, East Ethiopia. Int J Basic Clin Pharmacol. 2013;2(6):718. https://doi.org/10.5455/2319-2003.ijbcp20131209.
-
14.
Choudhury DK, Bezbaruah BK. Antibiotic prescriptions pattern in paediatric in-patient department gauhati medical college and hospital, Guwahati. J Appl Pharm Sci. 2013;3(8):144-8.
-
15.
Shankar PR, Upadhyay DK, Subish P, Dubey AK, Mishra P. Prescribing patterns among paediatric inpatients in a teaching hospital in western Nepal. Singapore Med J. 2006;47(4):261.
-
16.
Palikhe N. Prescribing pattern of antibiotics in pediatric hospital of Kathmandu valley. J Nepal Health Res Counc. 2004.
-
17.
Alakhali KM, Mohammad AAS. Prescribing Pattern of Antibiotics in Pediatric Patients in the Jazan Region, Kingdom of Saudi Arabia. RGUHS J Pharm Sci. 2014;4(3):120-4. https://doi.org/10.5530/rjps.2014.3.6.
-
18.
Ahmad A, Khan MU, Malik S, Mohanta GP, Parimalakrishnan S, Patel I, et al. Prescription patterns and appropriateness of antibiotics in the management of cough/cold and diarrhea in a rural tertiary care teaching hospital. J Pharm Bioallied Sci. 2016;8(4):335-40. [PubMed ID: 28216959]. [PubMed Central ID: PMC5314834]. https://doi.org/10.4103/0975-7406.199340.
-
19.
Bruzzese E, Giannattasio A, Guarino A. Antibiotic treatment of acute gastroenteritis in children. F1000Res. 2018;7:193. [PubMed ID: 29511533]. [PubMed Central ID: PMC5814741]. https://doi.org/10.12688/f1000research.12328.1.
-
20.
Blinova E, Lau E, Bitnun A, Cox P, Schwartz S, Atenafu E, et al. Point prevalence survey of antimicrobial utilization in the cardiac and pediatric critical care unit. Pediatr Crit Care Med. 2013;14(6):e280-8. [PubMed ID: 23823209]. https://doi.org/10.1097/PCC.0b013e31828a846d.
-
21.
Maltezou HC, Dedoukou X, Asimaki H, Kontou I, Ioannidou L, Mitromara K, et al. Consumption of antibiotics by children in Greece: a cross-sectional study. Int J Pediatr Adolesc Med. 2017;4(3):108-11. [PubMed ID: 30805511]. [PubMed Central ID: PMC6372488]. https://doi.org/10.1016/j.ijpam.2017.04.002.
-
22.
Ozkurt Z, Erol S, Kadanali A, Ertek M, Ozden K, Tasyaran MA. Changes in antibiotic use, cost and consumption after an antibiotic restriction policy applied by infectious disease specialists. Jpn J Infect Dis. 2005;58(6):338.
-
23.
Cotten CM, Taylor S, Stoll B, Goldberg RN, Hansen NI, Sanchez PJ, et al. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics. 2009;123(1):58-66. [PubMed ID: 19117861]. [PubMed Central ID: PMC2760222]. https://doi.org/10.1542/peds.2007-3423.
-
24.
Nylund CM, Goudie A, Garza JM, Fairbrother G, Cohen MB. Clostridium difficile infection in hospitalized children in the United States. Arch Pediatr Adolesc Med. 2011;165(5):451-7. [PubMed ID: 21199971]. [PubMed Central ID: PMC4683604]. https://doi.org/10.1001/archpediatrics.2010.282.
-
25.
Qureshi AI, Adil MM, Zacharatos H, Suri MF. Factors associated with length of hospitalization in patients admitted with transient ischemic attack in United States. Stroke. 2013;44(6):1601-5. [PubMed ID: 23632974]. https://doi.org/10.1161/STROKEAHA.111.000590.
-
26.
Fine MJ, Pratt HM, Obrosky D, Lave JR, McIntosh LJ, Singer DE, et al. Relation between length of hospital stay and costs of care for patients with community-acquired pneumonia. Am J Med. 2000;109(5):378-85. https://doi.org/10.1016/s0002-9343(00)00500-3.
-
27.
Chiang WC, Teoh OH, Chong CY, Goh A, Tang JP, Chay OM. Epidemiology, clinical characteristics and antimicrobial resistance patterns of community-acquired pneumonia in 1702 hospitalized children in Singapore. Respirology. 2007;12(2):254-61. [PubMed ID: 17298459]. https://doi.org/10.1111/j.1440-1843.2006.01036.x.
-
28.
Shenep JL, Flynn PM, Baker DK, Hetherington SV, Hudson MM, Hughes WT, et al. Oral cefixime is similar to continued intravenous antibiotics in the empirical treatment of febrile neutropenic children with cancer. Clin Infect Dis. 2001;32(1):36-43. [PubMed ID: 11112678]. https://doi.org/10.1086/317552.
-
29.
Moyo SJ, Gro N, Matee MI, Kitundu J, Myrmel H, Mylvaganam H, et al. Age specific aetiological agents of diarrhoea in hospitalized children aged less than five years in Dar es Salaam, Tanzania. BMC Pediatr. 2011;11:19. [PubMed ID: 21345186]. [PubMed Central ID: PMC3050719]. https://doi.org/10.1186/1471-2431-11-19.
-
30.
Wang V, Keefer M, Ong PY. Antibiotic choice and methicillin-resistant Staphylococcus aureus rate in children hospitalized for atopic dermatitis. Ann Allergy Asthma Immunol. 2019;122(3):314-7. [PubMed ID: 30529713]. https://doi.org/10.1016/j.anai.2018.12.001.
-
31.
Lutter SA, Currie ML, Mitz LB, Greenbaum LA. Antibiotic resistance patterns in children hospitalized for urinary tract infections. Arch Pediatr Adolesc Med. 2005;159(10):924-8. [PubMed ID: 16203936]. https://doi.org/10.1001/archpedi.159.10.924.
-
32.
Ghorashi Z, Ghorashi S, Soltani-Ahari H, Nezami N. Demographic features and antibiotic resistance among children hospitalized for urinary tract infection in northwest Iran. Infect Drug Resist. 2011;4:171-6. [PubMed ID: 22114509]. [PubMed Central ID: PMC3215345]. https://doi.org/10.2147/IDR.S24171.
-
33.
Mehrgan H, Rahbar M. Prevalence of extended-spectrum beta-lactamase-producing Escherichia coli in a tertiary care hospital in Tehran, Iran. Int J Antimicrob Agents. 2008;31(2):147-51. [PubMed ID: 18060745]. https://doi.org/10.1016/j.ijantimicag.2007.09.008.
-
34.
Rezai MS, Salehifar E, Rafiei A, Langaee T, Rafati M, Shafahi K, et al. Characterization of Multidrug Resistant Extended-Spectrum Beta-Lactamase-Producing Escherichia coli among Uropathogens of Pediatrics in North of Iran. Biomed Res Int. 2015;2015:309478. [PubMed ID: 26064896]. [PubMed Central ID: PMC4433631]. https://doi.org/10.1155/2015/309478.
-
35.
Kocak M, Buyukkaragoz B, Celebi Tayfur A, Caltik A, Koksoy AY, Cizmeci Z, et al. Causative pathogens and antibiotic resistance in children hospitalized for urinary tract infection. Pediatr Int. 2016;58(6):467-71. [PubMed ID: 26513232]. https://doi.org/10.1111/ped.12842.
-
36.
Warshawsky B, Hussain Z, Gregson DB, Alder R, Austin M, Bruckschwaiger D, et al. Hospital- and community-based surveillance of methicillin-resistant Staphylococcus aureus: previous hospitalization is the major risk factor. Infect Control Hosp Epidemiol. 2000;21(11):724-7. [PubMed ID: 11089657]. https://doi.org/10.1086/501718.
-
37.
Man SC, Primejdie DP, Sarkozi I, Popa A. Off-label and unlicensed prescribing in hospitalized children: prevalence and reasons. Farmacia. 2017;65:460-6.
-
38.
Thaulow CM, Berild D, Eriksen BH, Myklebust TA, Blix HS. Potential for More Rational Use of Antibiotics in Hospitalized Children in a Country With Low Resistance: Data From eight Point Prevalence Surveys. Pediatr Infect Dis J. 2019;38(4):384-9. [PubMed ID: 30882728]. https://doi.org/10.1097/INF.0000000000002106.