1. Background
Herpes simplex virus type 1 (HSV-1) is a member of alpha herpes virus family that is highly infectious (1). HSV-1 is transmitted via oral-oral contact (2) and respiratory secretions (3). It is estimated that the global prevalence of HSV-1 infection in all age group is 67% (3.7 billion people), in eastern meditation countries, this prevalence is 75% (4). Most cases of HSV-1 infection are acquired during childhood as an oral infection (5).
In clinical HSV-1 infection, prodromal signs last 1 - 3 days and include fever, reduced appetite, weakness, diarrhea, and in some cases, headache and nausea (6, 7). General oral pain due to multiple ulcers on keratinized and non-keratinized mucosa results in reduced food intake. This disease is self-limiting and recovery takes 10 to 14 days (8, 9).
After infection, the virus starts its movement through the axon of sensory nerves, which finally causes hidden infection in sensory ganglions (8). Some factors such as ultraviolet radiation, shock, stress, fever, trauma and menstruation trigger the reactivation of HSV in sensory ganglions (10, 11). As a result, the virus moves toward mucus membranes or the skin, a place where the virus can act as a pathogen for epithelial cells (12) and results in recurrent infection in the form of vesicles and topical ulcers (13) or asymptomatic shedding of virus into saliva and oral secretions (14).
Asymptomatic release of the virus is not associated with systemic signs and symptoms. There is controversy over the prevalence of asymptomatic shedding occurring after dental treatments (15-17). In some cases, HSV activation after dental procedures is very severe and can extend to the periphery of the usual site of recurrence (18, 19).
Asymptomatic shedding promotes the risk of cross-infection not only to other patients but also to dental staff (20, 21). This is especially important in immunocompromised patients and pregnant women (22, 23). Therefore, the evaluation of asymptomatic HSV-1 shedding induced by dental treatment is important. In this study, we sought to determine HSV-1 oral shedding before and after dental treatments (fixed prosthesis and root canal treatment). In addition, we sought to investigate the probable effect of dental treatment on HSV-1 shedding or recurrence of HSV-1 infection.
2. Methods
Prior to the study, we obtained the approval of the Ethics Committee of Tehran University of Medical Sciences. The patients were informed of the study objectives and signed the informed consent forms.
2.1. Patients
The participants included the patients presenting to the Faculty of Dentistry of Tehran University of Medical Sciences for conventional dental care. All the patients had a history of recurrent herpes labialis (RHL). The patients were selected randomly from adults, regardless of their gender. All the patients were from the same geographical region and had comparable socioeconomic status. The exclusion criteria included immunocompromised patients, pregnant women, women in their menstrual cycle, patients presenting RHL during or seven days prior to sample collection, patients with a history of head and neck trauma, patients experiencing mental stress or acute exposure to sunlight and patients used antiviral agents during and one month prior to the study. From the 22 selected patients (m = 6, f = 16, mean age 34.57 years), 11 subjects underwent root canal therapy and the remaining 11 patients received fixed dental prosthesis treatment (Table 1).
| Characteristic | Endodontic | Fixed Prosthesis |
|---|---|---|
| Age, years | ||
| Median | 35.6 | 33.54 |
| Range | 20 - 50 | 19 - 50 |
| Gender | ||
| Female, No. (%) | 9 (81.8) | 7 (63.6) |
| Male, No. (%) | 2 (18.1) | 4 (36.3) |
2.2. Saliva Samples Collection
First, saliva samples were taken from the patients just before starting the treatment. The patients were asked to keep their saliva in their mouth for five minutes and then, spit each 60 second until 3 ml of saliva was collected in a sterile container (spitting method). The second round of sample collection was three days after treatment using the same protocol. DNA was isolated from saliva samples using Promega DNA Purification Kit (Promega Corporation, Madison, WI, USA) according to the manufacturer’s protocol and subjected to polymerase chain reaction (PCR). The samples were processed in three different physical environments at each step of the procedure to prevent contamination, namely (1) nucleic acid extraction area, (2) pre-PCR staging area, and (3) DNA amplification area, with distribution of the PCR product and detection of DNA fragments after PCR. At least three negative controls (Milli-Q water) were used per assay, including interleaving of the samples. The samples obtained from DNA extraction were submitted to amplification reaction of human β-globin DNA for the evaluation of their viability. A set of primers was designed against the HSV-1 UL42 gene (Table 2).
| Virus | Primers | PCR Program |
|---|---|---|
| HSV-1 | F: 5’-GCCAGCGAGACGCTGAT-3’ | 94°C/60 s; 40°C/60 s; 72°C/60 s |
| R: 5’-ACGCAGGTACTCGTGGTGA-3’ |
a F, forward primer; R, reverse primer.
2.3. Polymerase Chain Reaction (PCR)
PCR was performed using 10 µL of 10 x PCR buffer, 4 µL of MgC (25 mM), 8 µL of dNTPs (2.5 mΜ), 2 µL of primer HS13 (50 Pmol/µL), 2 µL of primer HS14 (50 Pmol/µL), 1 µL of Taq DNA polymerase (5 U/µL), Taq µL of DNA sample and 63 µL of H2O. The UL42 gene was amplified with a 1-min denaturation step at 94°C, a 1-min annealing step at 40°C and 1 minute elongation at 72°C for 35 cycles. The PCR products were run on 2% agarose gel, stained with ethidium bromide and visualized under ultraviolet (UV) light. Positive controls of a herpesvirus culture were also included, and the negative control was water (Figure 1).
3. Results
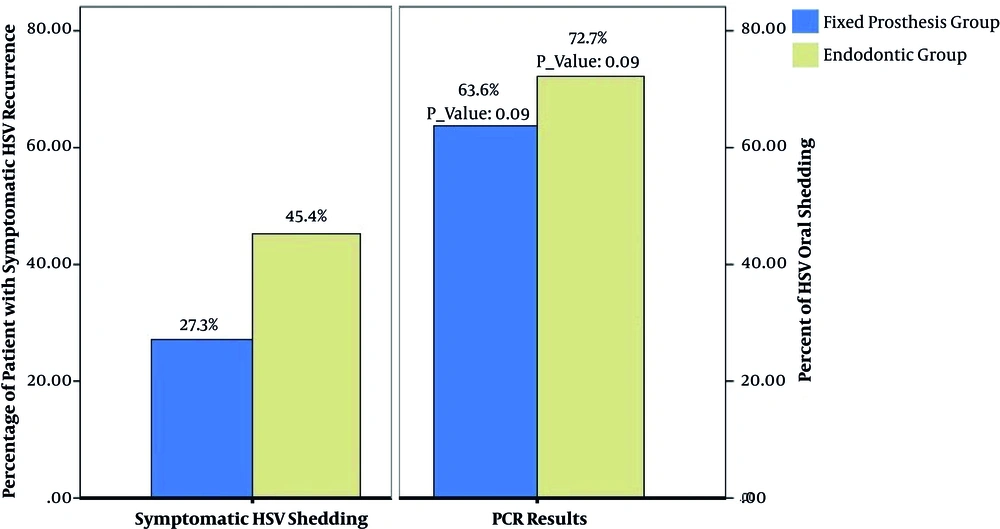
In this cross-sectional study, 22 patients were enrolled, of whom 16 subjects were female and 6 were male. The patients’ age ranged from 19 to 50 years old, and none of them had history of dental procedure-related RHL (DIRR). Eight of the 11 patients (72%; P = 0.09) undergone root canal therapy and seven of 11 patients (63%; P = 0.09) received fixed partial prosthodontics treatment had asymptomatic shedding of HSV-1. Five (45.45%) subjects from the root canal therapy group and 3 (27.3%) subjects from the fixed partial prosthodontics treatment group showed clinical signs of RHL or intraoral recurrent herpes infection within seven days of treatment (Figure 2). The frequency of viral shedding in patients under endodontic treatment was higher than that in the fixed prosthodontic treatment group, which could probably be due to pain and central nervous system excitement accompanied with endodontic treatment.
4. Discussion
Preliminary infection with HSV-1 can cause several health problems (24, 25). It seems that symptoms of HSV-1 shedding present three days after infection (19, 26). Thus, we collected patient specimens three days following dental treatment. PCR is an accurate molecular diagnostic tool that is very sensitive for the identification of HSV infections (27-29).
Immunoglobulins present in saliva are one of the limitations in the identification of HSV infections by cell culture, while they cannot influence PCR results (21, 30). HSV infection can be detected in about 25% of patients using viral culture and cytological methods (31). On the other hand, HSV-1 infection can be detected in 90% of patients using PCR (32, 33).
There are several studies on recurrent intraoral or labial herpes following dental treatment (20, 33). These types of studies are important because HSV shedding can cause disease transmission to dental staff and HSV keratitis and other complications of HSV infections (34, 35). In addition, HSV shedding in immunocompromised patients may induce disseminated and potentially fatal diseases. Thus, regarding increasing HS viral load after dental treatment, considering anti-viral medications is recommended (32, 36). Miller et al. stated that at least 70% of the population shed HSV-1 asymptomatically at least once a month, and many individuals appear to shed HSV-1 more than six times a month. Also, traumatic oral procedures increase the likelihood of HSV shedding in the oral cavity (33).
Hyland concluded that shedding of HSV-1 in the oral cavity not only increases by direct surgical trauma but also appears to be common in migraine and temporomandibular diseases (TMD) patients attending for general dental treatment. Thus, pain or pain-induced stress, as well as anxiety associated with dental treatment may also be a risk factor for asymptomatic shedding in specific seropositive patients receiving dental treatment (20). The study by Ramchandani et al. (14) indicated that oral reactivation of HSV-1 in comparison with HSV-1 shedding in tears and nasal mucosa is more common and usually asymptomatic. Frequent oral shedding of HSV-1 may increase the risk for transmitting the virus to oral mucosa (14); these findings confirm our results.
Asymptomatic oral HSV shedding was reported by El Hayderi et al., which was line with our findings. However, severe RHL after dental treatment was not reported in that study, whereas in our study 45.45% of root canal therapy patients and 27.3% of fixed prosthodontics patients showed RHL after dental treatment. El Hayderi et al. suggested that nerve damage due to dental treatment causes HSV-1 reactivation and concluded that oral HSV shedding may be related to invasiveness of dental procedure, but further studies are needed on this issue (2, 37).
De Santana Sarmento et al. and Caliento et al. concluded that kidney transplant recipients (adults and children, respectively) excreted herpes viruses more often than controls, especially HSV-1 and Epstein-Barr virus, with salivary shedding of herpes viruses being more frequent in patients with recent kidney transplantation (38, 39). As we have argued elsewhere, these studies confirmed higher HSV-1 shedding in immunocompromised patients in comparison with healthy controls. The frequency of viral shedding in patients under endodontic treatment was higher than those received fixed prosthodontic treatment, which is probably due to pain and central nervous system excitement accompanied with endodontic treatment. This finding indicates that pain and pain-induced stress can activate herpes virus.
4.1. Conclusions
In this study, recurrent HSV-1 infection in 72% of the root canal and 63% of the fixed dental prosthesis patients, either symptomatic or asymptomatic, was present, which is consistent with previous reports (32, 40, 41). Asymptomatic viral shedding can be observed not only in acute infections, but also in the prodromal stage, even before clinical manifestations (2, 33). High levels of stress and dental treatment-related traumas can also elevate the risk of asymptomatic shedding in saliva (20). High levels of cortisol released during pain is one of the most important activators of the stress system that can instigate recurrent viral infection (42). Therefore, immunomodulation molecules that are activated due to dental treatment-related pain can cause recurrent HSV-1 infections. In addition, specific molecular signals such as adrenalin, IL-6, cAMP, glucocorticoids and prostaglandins are released during stress (42, 43).
Based on our results, the risk of viral infection transmission to dentistry staff and other patients should be strongly considered. Therefore, prophylactic acyclovir treatment before treating symptomatic patients with a historyof DIRR is crucial. Overall, our results demonstrated the strong impact of dental treatment such as endodontics and prosthodontics treatment on increased HSV viral shedding in the oral cavity. An important issue is that PCR is a qualitative laboratory method; thus, determination of viral load using real time PCR is recommended.