1. Background
Diazinon (O,O-Diethyl O-[4-methyl-6-(propan-2-yl)pyrimidin-2-yl] phosphorothioate) is a well-known organophosphate pesticide that was invented during World War II and commercially introduced in 1952 (1). Diazinon acts through chemical binding to the acetylcholinesterase (AChE) enzyme to inhibit it. The World Health Organization (WHO) classifies diazinon as class II “moderately toxic” chemicals (2). Relative water solubility (40 mg per liter) and moderate mobility and persistence of diazinon raise concerns about the potential contamination of groundwater by this chemical. Diazinon can be detected in the environment as long as four months after application (3). Diazinon accounts for more than half of the insecticides used in Iran (4).
Much evidence suggests that exposure to diazinon may cause neurotoxicity, cardiotoxicity, and vascular toxicity (5). Furthermore, the elevated incidence of lung cancer among licensed diazinon applicators was observed in a recent study (6).
Oxidative stress caused by the metabolism of pesticides may be responsible for permanent damage to cellular mechanisms (7). Several studies have used various antioxidants to alleviate pesticide-induced oxidative stress in animal models (8). Recently, Al-Attar et al. demonstrated that pretreatment with grapeseed oil had protective effects against diazinon toxicity in rats, which may be due to the antioxidant role of its constituents (9).
Sambucus genus belongs to Caprifoliaceae and includes 18 species all over the world. Four species of the genus are growing in Iran. Of these species, S. ebulus extensively grows in moist grasslands and forest margins on the Northern coast of Caspian Sea. It has been reported that S. ebulus extract has anti-hemorrhoid, antiprotozoal, and antibacterial properties against Helicobacter pylori and it is effective in the treatment of burns, infectious wounds, edema, and eczema (10). In addition, significant antioxidant activities of S. ebulus extracts have been reported (11, 12). Recently, good antiemetic, neuroprotective, antidepressant, anti-inflammatory, and wound healing activities of this plant have been proven (13, 14).
2. Objectives
The aim of this study was to determine the effect of diazinon on the expression levels of two genes involved in phase I and phase II xenobiotic metabolism (cyp1a1 and gstm1, respectively) and two genes involved in DNA damage sensing and apoptosis (pmaip1 and diras3, respectively) in MRC-5 cells. Furthermore, the protective effect of the methanol extract of S. ebulus leaves was examined against diazinon toxicity in the MRC-5 cell line.
3. Methods
3.1. Materials and Method
High-purity diazinon (> 98%) was purchased from Sigma-Aldrich. All cell culture reagents were obtained from GibcoTM. MTS assay materials were provided by Promega (Madison, WI, USA) and kits for qPCR experiments were supplied by Qiagen (Germany).
3.2. Cell Culture
The human fetal lung fibroblast cell line (MRC-5) was purchased from the National Cell Bank of Iran (NCBI, Code C125) and cultured in Dulbecco’s modified eagle medium (DMEM) supplemented with 10% (vol.) fetal bovine serum (FBS), 1% (vol.) of 25 mM Glutamax, and 1% (vol.) non-essential amino acids (NEAA). Cells were maintained at 37°C in a humidified atmosphere containing 5% CO2. Cell culture media were refreshed every two days.
3.3. Plant Materials
Sambucus ebulus (L.) leaves were collected from Sari, Iran. Leaves were identified by Dr. Bahman Eslami and a voucher (No. 1395) was deposited at the Herbarium of Sari School of Pharmacy. Plant materials were air-dried and then coarsely grounded to about 2 - 3 mm. Leaves were extracted by the maceration method using methanol as the extracting solvent for 72 hours. The resulting extract was concentrated over a rotary vacuum (about 35°C) until a crude solid extract was obtained that was then freeze-dried for complete solvent removal.
3.4. Diazinon Cytotoxicity Assessment
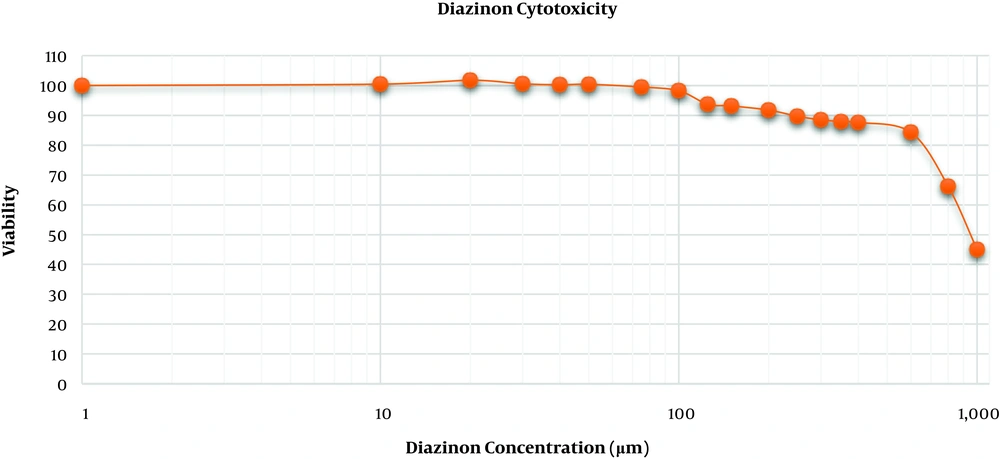
To determine the toxic concentration of diazinon for MRC-5 cells, diazinon was diluted using cell culture grade DMSO to prepare a stock solution of 1 M that was stored in a light-protected container at room temperature. MRC-5 cells were seeded in 96-well cell culture plates at a density of 7000 cells/well and incubated for 48 hours. After that, the plated cells were washed using DPBS and treated with a range of diazinon concentrations (10 to 1000 μM) with three replicates for 24 hours. Cells were serum-starved during treatments. After 24 hours, 20 μL of the MTS/PMS solution was added to 200 μL of each well and optical density was recorded at 492 nm after 4 hours. The 50% inhibitory concentration (IC50) of cell proliferation was directly determined from the dose-response curve.
3.5. Leaf Extract Cytotoxicity Assessment
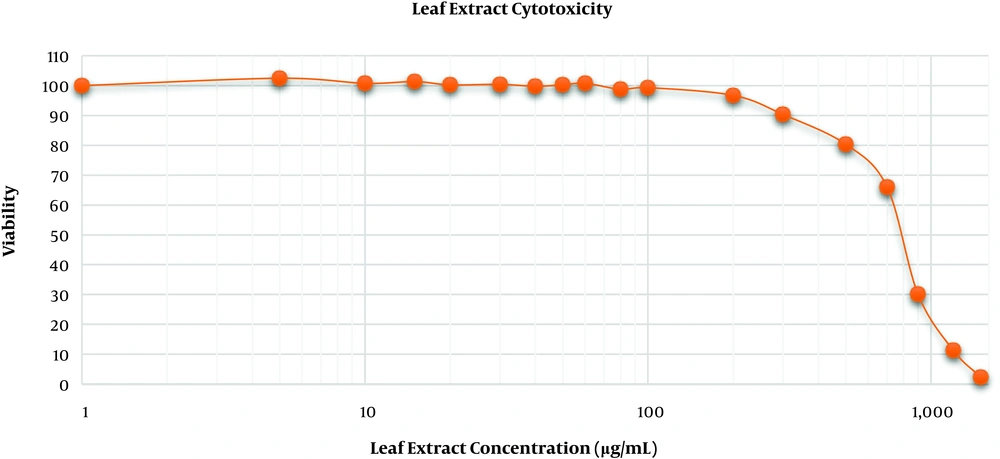
The toxic and inhibitory concentration of S. ebulus extract was determined by the MTS assay as mentioned above. The extract was dissolved using high-purity water and 4% tween 80 to a stock solution of 150 mg/mL and stored at 4°C. MRC-5 cells were treated with a range of extract concentrations (5 to 1500 μg/mL) and the toxic and inhibitory concentrations of the extract were determined using the dose-response curve.
3.6. RNA Extraction and Real-Time qPCR
To assess the effect of diazinon alone or in combination with the extract on gene expression levels, MRC-5 cells were cultured in 6-well cell culture plates at a density of 15 × 104 cells per well for 48 hours. After seeding, the cells were exposed to the vehicle (0.01% DMSO) and diazinon concentrations of 5, 10, 25, 50 and 100 μM. For extract treatment, cells were treated with 50 μM of diazinon in combination with 0 (DPBS), 5, 10, 20, 40, and 80 μg/mL of the leaf extract for 24 hours. To mimic the in vivo condition of exposure to organophosphate pesticides, we used the in vitro concentrations of diazinon in a range of 5 - 100 μM that had demonstrated as the most appropriate concentrations (15). Moreover, according to the results of gene expression analysis in response to diazinon, we used 50 μM of diazinon solution combined with a range of extract concentrations (5 - 80 μg/mL). Total RNA was extracted using the RNeasy Mini Kit (Qiagen) and complementary cDNA was synthesized using the QuantiNova Reverse Transcription Kit (Qiagen) from 1 μg of freshly prepared RNA. The quality and quantity of extracted RNA were evaluated by Nanodrop (Thermofisher, USA) and 1% agarose gel electrophoresis. All experiments were done in triplicate.
Real-time qPCR was performed in Rotor-gene 6000 (Corbett) using the QuantiNova SYBR Green PCR kit (Qiagen) in a final volume of 10 μL reaction mix. Gapdh was used as the reference gene and relative gene expression levels compared to control cells were evaluated by the comparative ΔΔCT method as described elsewhere (16). The reference and target gene primers are shown in Table 1.
| Gene Name | Direction | Sequence (5’ to 3’) | Amplicon Size (Base Pair) |
|---|---|---|---|
| gapdh | Forward | TTCCGTGTTCCTACCC | 167 |
| Reverse | GCTGTTGAAGTCGCAG | ||
| gstm1 | Forward | GTACACGATGGGGGACGCTC | 178 |
| Reverse | TTCTGTCTCCCCACACAGGTTG | ||
| cyp1a1 | Forward | TCAGTACCTCAGCCACCTCCAAG | 200 |
| Reverse | GGTCAGCATGTGCCCAATCAGA | ||
| diras3 | Forward | GTCGGAATATAAAACCGCGGAGGAG | 132 |
| Reverse | GGCAGCAGGAGACCCCTTTC | ||
| pmaip1 | Forward | TCCTGCAGCTGTCCGAGGT | 158 |
| Reverse | GCACACTCGACTTCCAGCTCTG |
3.7. Statistical Methods
Data analysis was performed using SPSS V. 24 for Windows. Two-way analysis of variance (ANOVA) was used to evaluate the significance of differences in gene expression levels between the treated groups and the control group. In addition, the Dunnett test was used for further statistical comparisons. The data were expressed as means ± standard deviation and P values of less than 0.05 were considered significant.
4. Results
Cytotoxicity assessment of diazinon disclosed that no significant inhibition in the MRC-5 cell proliferation occurred at concentrations below 100 μM. However, diazinon concentrations of more than 125 μM dramatically inhibited the MRC-5 cell proliferation and the concentration of 950 μM decreased MRC-5 cells viability to 50% (Figure 1).
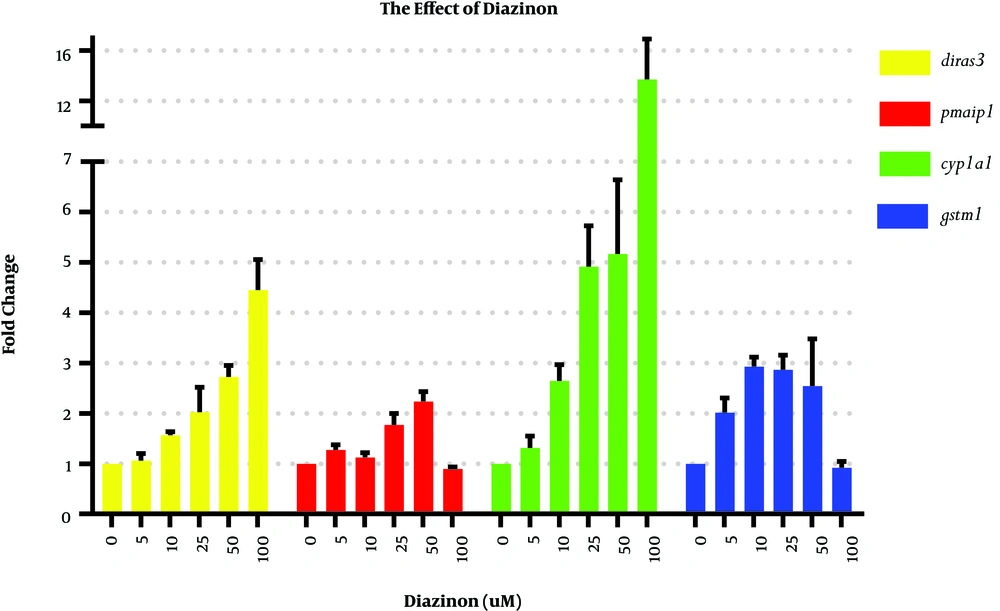
To assess the influence of diazinon on cyp1a1, gstm1, pmaip1, and diras3 mRNA expression levels, we exposed the MRC-5 cell line to 5, 10, 25, 50, and 100 μM of diazinon for 24 hours. DMSO-treated cells were considered as the control cells (Figure 2). The significant Dunnett’s test results of gene expression analysis in Figure 2 are shown in the supplementary file (Appendix 1).
As shown in Figure 3, the viability of MRC-5 cells exposed to 5 to 80 μg/mL of the extract did not significantly differ from the viability of control cells but the extract concentrations of more than 100 μg/mL inhibited the cell growth and the IC50 of the extract was determined to be about 780 μg/mL. Accordingly, in the rest of the experiments, 80 μg/mL of the extract was used as the plausible concentration.
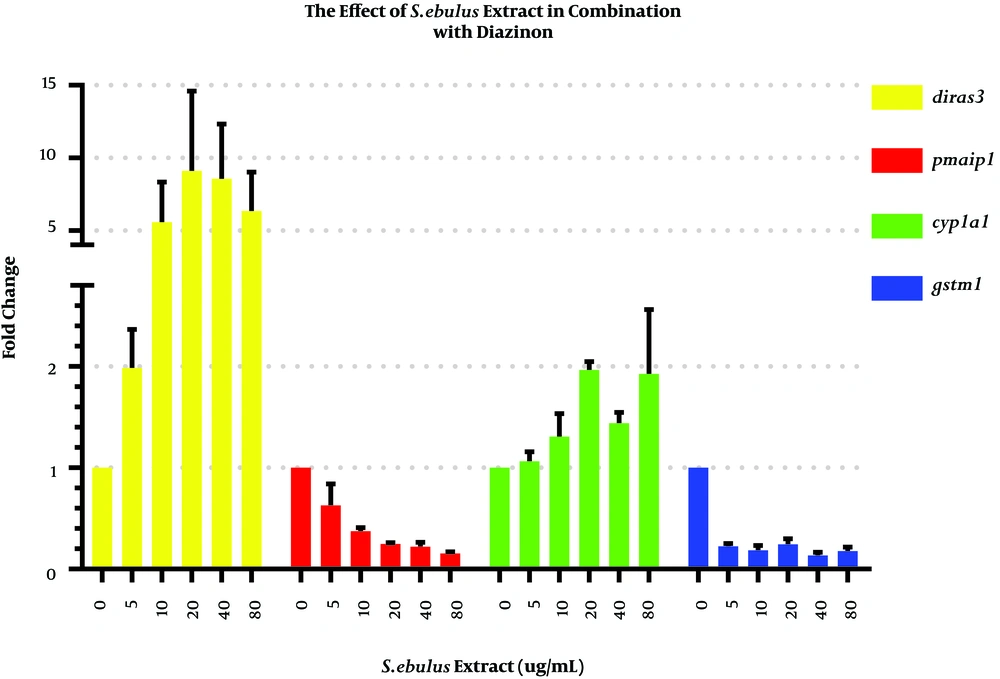
To investigate the effect of the extract in combination with diazinon on cyp1a1, gstm1, diras3, and pmaip1 mRNA expression levels, we exposed MRC-5 cells to the mixtures of 50 μM of diazinon with 5, 10, 20, 40 and 80 μg/mL of the extract or DPBS as the control group (Figure 4). The results of Dunnett’s test analysis for gene expression are shown in the supplementary file (Appendix 2).
5. Discussion
A major human health concern about commonly used agricultural pesticides such as organophosphate pesticides seems to be due to their genotoxic effects (17). The elevated risk of cancer due to pesticide exposure indicates the lack of adequate knowledge of pesticides’ metabolisms and carcinogenicity and the need for a medicine or medical herb to decrease the adverse effects of pesticide metabolites.
The results of the current study indicated that the cyp1a1 gene was strongly induced by the pesticide, as expected. Elisson et al. showed that the activation of diazinon is primarily catalyzed by the CYP1A1 enzyme (18). We found that the minimum concentration of diazinon to significantly induce the cyp1a1 expression was 25 μM that could upregulate the cyp1a1 expression by 4.91 folds (P = 0.029).
As exposure to agricultural pesticides can induce oxidative stress in cells (19) and diras3 has a key role in the initiation of apoptosis, diazinon treatment elevated diras3 mRNA levels compared to the control group, as expected. Diazinon concentrations of more than 25 μM significantly elevated the diras3 mRNA levels compared to control cells (P < 0.05 for all comparisons).
In the current study, we found that the upregulation of gstm1 mRNA levels occurred in almost all treatment conditions except for the cells treated with 100 μM of diazinon. Previous studies illustrated that organophosphate pesticides such as chlorpyrifos are the inducers of gstm1 (17), which is in accordance with our results.
As shown in Figure 2, only could 25 and 50 μM concentrations of diazinon significantly induce pmaip1 mRNA (by 1.7 and 2.2 folds, respectively). These results show that diazinon is not a potent inducer of pmaip1. Contrary to our results, previous studies demonstrated that exposure to agricultural pesticides such as paraquat (a kind of herbicide) resulted in the elevation of apoptotic markers such as pmaip1 (20).
As observed for MRC-5 cells exposed to diazinon, the cyp1a1 mRNA levels were induced by a mixture of 50 μM of diazinon and 20 or 80 μg/mL of the extract by 1.96 and 1.92 folds, respectively. It has been demonstrated that extracts obtained from other herbs such as K. parviflora significantly enhanced CYP1A1 activity. Thus, further investigation should determine the effects of herbs in combination with pesticides on cellular mechanisms (21).
The diras3 mRNA levels in response to 20 or 40 μg/mL of the extract in combination with 50 μM of diazinon were significantly elevated by 9 and 8.5 folds (P = 0.033 and 0.47, respectively). Contrary to our imagination, the results illustrated that the extract itself had genotoxic effects and might induce oxidative stress. Nevertheless, other studies pointed to the neuroprotective role of S. ebulus fruit extract against oxidative stress (14). It is possible that a component of the leaf extract is the reason for diras3 induction that does not persist in the fruit extract.
Conversely, the mixture of the extract and pesticide strongly decreased the gstm1 mRNA levels in treated cells compared to controls (P < 0.05 for all comparisons). Recent studies showed that serum glutathione levels increased in the presence of diazinon and significantly decreased in the presence of a mixture of olive oil and diazinon, suggesting that oils or extracts of some plants or herbs may have a high capacity of antioxidant activity (9).
Surprisingly, the pmaip1 gene transcriptionally decreased in MRC-5 cells treated with the mixture of the extract and pesticide. Research mainly shows that plant extracts can induce pmaip1 expression. For instance, Elkady demonstrated that the crude extract of Rhazya stricta, a medical herb with free radical scavenging properties, induced apoptosis via pmaip1 overexpression in lung cancer cell line (22).
5.1. Conclusions
In summary, our findings indicated that diazinon elevated the expression of phase I and phase II detoxification genes. In addition, we illustrated the effect of a medical herb extract in combination with a commonly used agricultural pesticide on MRC-5 cells. The mixture of the extract and diazinon upregulated the expression of diras3, implying that a component of the extract had genotoxic effects. The extract effectively scavenged free radicals and downregulated the gstm1 expression. Further investigations need to be performed to determine the toxic and safe fractions of the extract.