1. Background
Malaria remains a major public health problem in tropical regions. It is caused by different species of Plasmodium belonging to the haemosporidian order and is transmitted by female anopheles mosquitoes during a blood meal from a human (1, 2). It can be harmful to social and economic development, and is also one of the leading causes of morbidity and mortality (3). According to the annual report by the World Health Organization (WHO) in 2014, approximately 198 million cases of malaria occurred globally in 2013 and the disease led to 584,000 deaths (4).
In Iran, the distribution and transmission of malaria are mostly limited to three Provinces of Sistan and Baluchistan, Hormozgan, and Kerman, locatedin the southern and southeastern areas of Iran (5, 6). They approximately share common frontiers (90%) with Pakistan and Afghanistan, where the malaria is endemic that can be one of the risk factors for importation of malaria into Iran (7).
The number of malaria cases in Sistan and Baluchistan that shares a wide boundary with Pakistan were 8,657 people (53% of all cases in Iran) in 2007 (8). Also, Sistan and Baluchistan has regional climate variety and proper geographical features for the presence of different species of anopheles as a carrier of malaria (9). Therefore, successive consideration, especially epidemiologically, is needed to address this issue. Both symptomatic and asymptomatic patients play an important role in the transmission of the infection. It has reported that many asymptomatic people were recognized as a malaria case in areas with a high and moderate load of malaria transmission (10), therefore, malaria elimination programs should be highly considered (11). To achieve malaria elimination, as a global target, early diagnosis of the diseases, parasite rates, serious surveillance, and epidemiological studies are crucial (4, 12).
The epidemiology of malaria has been poorly studied in asymptomatic individuals, especially in boundary soldiers (4).
2. Objectives
Therefore, this study aimed at evaluating the prevalence of malaria in veterans.
3. Methods
3.1. Study Design
In this cross-sectional study, a total of 300 volunteers were selected from a garrison in Zahedan City, Sistan and Baluchestan, Southeast of Iran, during the spring and summer of 2017. Blood samples (2 cc) were obtained from each case and collected into the tubes containing EDTA (as an anti-coagulant), and simultaneously, the questionnaires were completed by the subjects. Then, the samples were examined by microscopic examination and nested PCR method.
All participants had not travelled to the endemic area for three months before sampling and also, had not received anti-malarial drugs for three weeks before sampling.
3.2. Microscopy Technique
Thick and thin blood smears were prepared from each sample separately, and after staining via Giemsa staining method (2), they observed under the microscope in the research center of the Bualisina Hospital, Zahedan.
3.3. Nested PCR Technique
DNA was extracted from whole blood samples using DynaBio blood/tissue DNA extraction mini kit (cat. no.: L-94044). The nested PCR method was carried out to detect Plasmodium spp. using the primers, such as rPLU6 and rPLU5 and rPLU3 and rPLU4 (Table 1) (13, 14).
| Primers | Sequence |
|---|---|
| rplu5 | 5’-CTTGTTGTTGCCTTAAACTT-3’ |
| rplu6 | 5’-TTAAAATTGCAGTTAAAACG-3’ |
| rPLU3 | TTT TTA TAA GGA TAA CTA CGG AAA AGC TGT |
| rPLU4 | TAC CCG TCA TAG CCA TGT TAG GCC AAT ACC |
| rFAL.1 | 5’-TTAAACTGGTTTGGGAAAACCAAATATATT-3’ |
| rFAL.2 | 5’-ACACAATGAACTCAATCATGACTACCCGTC-3’ |
| rVIV.1 | 5’-CGCTTCTAGCTTAATCCACATAACTGATAC-3’ |
| rVIV.2 | 5’-ACTTCCAAGCCGAAGCAAAGAAAGTCCTTA-3’ |
| rMAL.1 | 5’-ATAACATAGTTGTACGTTAAGAATAACCGC-3’ |
| rMAL.2 | 5’-AAAATTCCCATGCATAAAAAATTATACAAA-3’ |
| rOVAL.1 | 5’-TGTAGTATTCAAACGCAGT-3’ |
| rOVAI2 | 5’-TATGTACTTGTTAAGCCTTT-3’ |
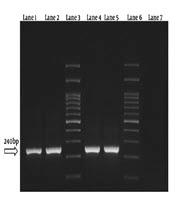
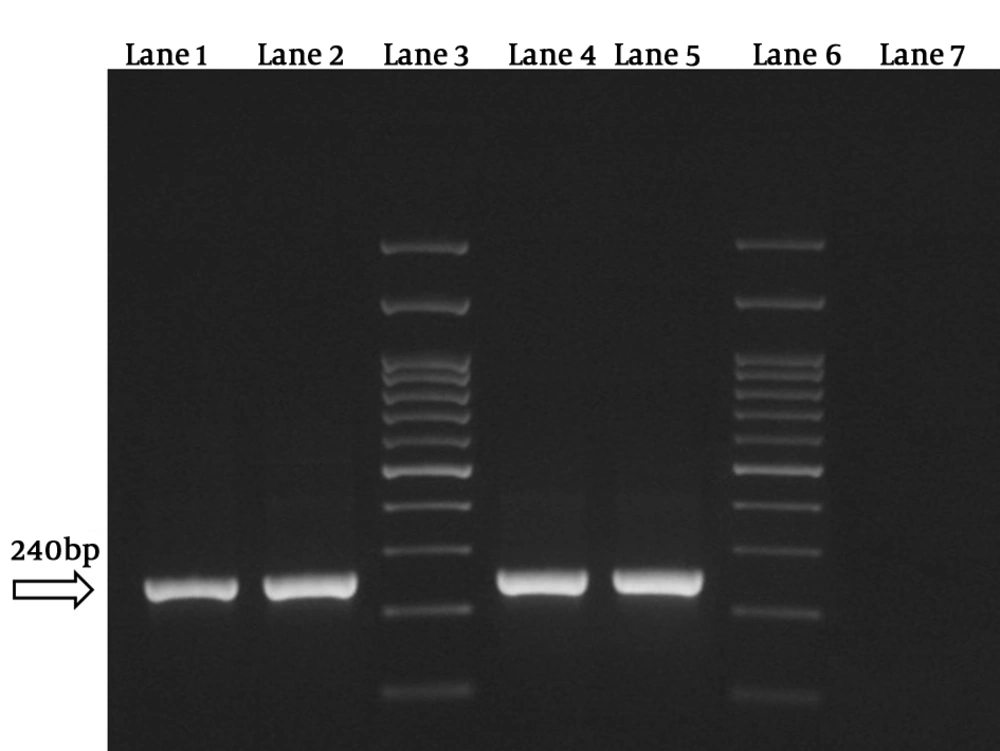
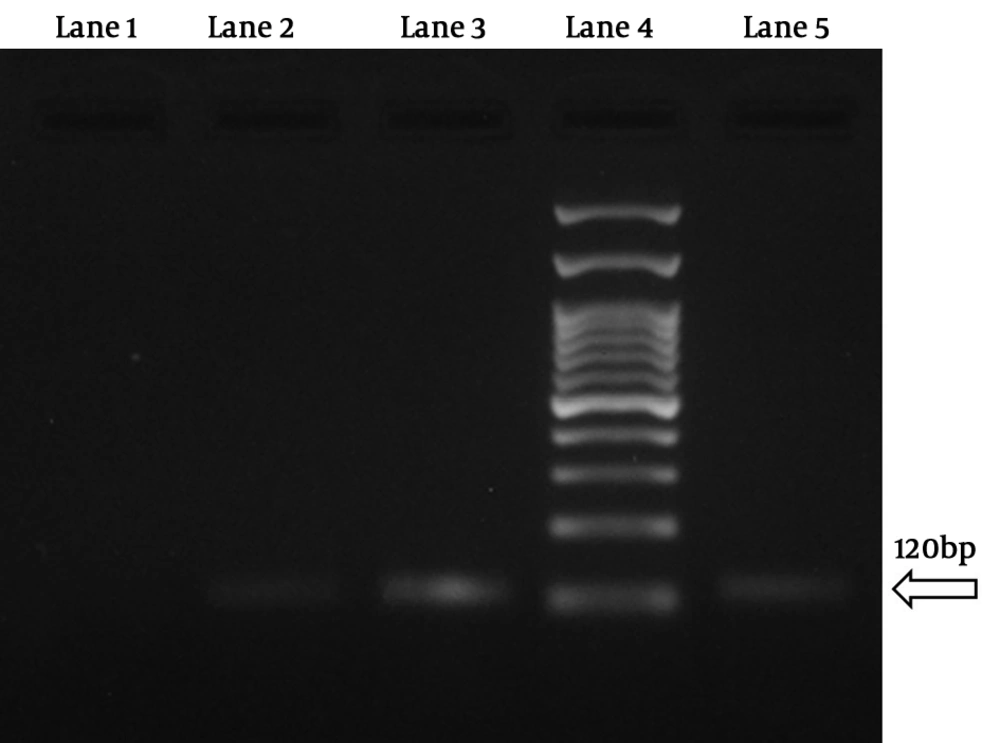
In the first step of reaction, the positive samples for Plasmodium spp. were revealed by observation of the 240 bp band and in the second step of reaction, the different species were detected. The used primers were as follows: rFAL 1 and 2, rMAL 1 and 2, rVIV 1 and 2, and rOVA 1 and 2 (Table 1) (13, 14).
The amplification program for nested 1 PCR was as follows: 95°C for 5 min, and then 25 cycles of 94°C for 60 s, 58°C for 60 s, and 72°C for 2 min and the final elongation was conducted at 72°C for 7 min. At the end of reaction, the temperature was decreased to 4°C. The thermocycler temperature and time were the same for nested 2 PCR, but it was repeated for 30 cycles.
4. Results
4.1. Microscopy Technique
No Plasmodium parasites were detected.
4.2. Nested PCR Technique
The results showed that 4 samples (1.3%) were positive for P. vivax. Besides, no Plasmodium falciparum and Plasmodium malariae were detected by this technique (Figures 1 and 2). Three samples were native to Sistan and Baluchestan and another one was native to Kerman.
5. Discussion
According to the results of this study, of 300 studied samples, 4 samples (1.3%) had asymptomatic malaria. Additionally, all positive cases were P. vivax. Three of them were native to Sistan and Baluchistan and another one was native to Kerman. These results are in agreement with the results of studies from 2005 to 2017, in indicating a gradual decrease in the incidence rate of malaria. They also reported no transmission via anopheles mosquitoes in a particular geographical regions. Based on these studies, the annual incidence rates of malaria were 469, 345, and 359 (per 100,000 persons) in three successive years (2005 - 2008), respectively (15). Norouzinezhad et al. (8) found the annual incidence rates of 89.9, 43.9, 38.3, and 36.6 (per 100,000 persons) over 4 years, respectively. In Iran, only 330 malaria cases were reported in 2015, which decreased by 50% than 2014 (16). According to the reports by the WHO, malaria is about to be eradicated in Iran (17).
Therefore, a low rate of prevalence of infection in endemic area indicated that its prevalence has decreased during last years. In addition, by joining Iran to the malaria pre-elimination program since 2009, several activities, such as barrier strategies, following up recent cases, and providing appropriate treatment for patients in areas where malaria is common have been considered. Therefore, it seems that the infection has been controlled successfully, and also based on our study done on soldiers in one of the endemic area of Iran, it has managed using military control programs in military members. Besides, malaria control contributes to the socio-economic progression.
Positive cases had no symptoms of malaria and might be considered as silent carriers. The silent carrier should be concerned more, because they have a serious role in the transmission of infection (11).
In the present study, the nested PCR method showed high sensitivity and also, it could detect both mixed infectious and parasite species. Several studies have shown that the sensitivity and specificity of molecular techniques (PCR), were higher than the microscopic examination (18, 19). Zakeri et al. studied the outbreak of malaria in the Southeastern Regions of Iran by nested PCR technique. One hundred and twenty samples targeting ssrRNA gene were evaluated by PCR methods and P. vivax, P. falciparum, and a mixed infection by P. vivax and P. falciparum were detected in 59%, 10%, and 28.4% of the patients, respectively (20). Turki et al. (2) studied the prevalence of malaria in 200 healthy volunteers in the endemic area of Iran by nested PCR, microscopic examination, and rapid diagnostic techniques (RDT). No Plasmodium parasites were detected in the subjects using microscopic examination and RDT, but 3 samples (1.5%) of P. vivax were found by nested PCR (2). Zakeri et al. reported microscopic technique as the gold standard for diagnosis of malaria, because of its convenience, effectiveness and possibility to use in medical settings. However, it is associated with potential errors to diagnose parasite species and asymptomatic cases of malaria. Also, it is difficult to diagnose the mixed Plasmodium infection or recognize the disease after drug consumption (with a low rate of parasitemia) by the microscopic technique.
Accurate diagnosis of malaria species is effective to cure malaria and reduce carriers in the general population (21).
In this study, all positive cases were detected by nested PCR. Using an accurate, accepted, and sensitive technique is essential for the diagnosis of malaria in elimination programs, otherwise asymptomatic cases might be missed in this phase (18). Therefore, although microscopic examination is cost-effective and convenence, but is a labour-intensive technique and requires professional personnel to detect the asymptomatic patients related to low parasitemia. Therefore, microscopic examination is associated with some limitations in the diagnosis of malaria (22) and using the nested PCR technique is preferred.
Based on the results, only P. vivax was detected, which needs more assessments to reveal the associated factors, such as the presence of anophel species (23) as the carriers or on the contrary, the lack of carriers, such as P. falciparum and another species. In addition, it should be investigated that how the infection has remained in the area, and also whether it is introduced or imported malaria. It is possibly linked to the manifestation of diseases, as it is often intense in P. falciparum, leading to symptomatic cases that are diagnosed fast or easy and cosequently result in the early treatment and elimination in the area.
5.1. Conclusions
A low prevalence (1.3%) of malaria indicated that it has decreased during the last years and using the nested PCR technique is suggested to diagnose malaria in asymptomatic patients.