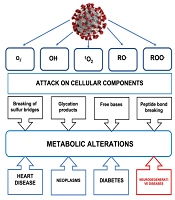
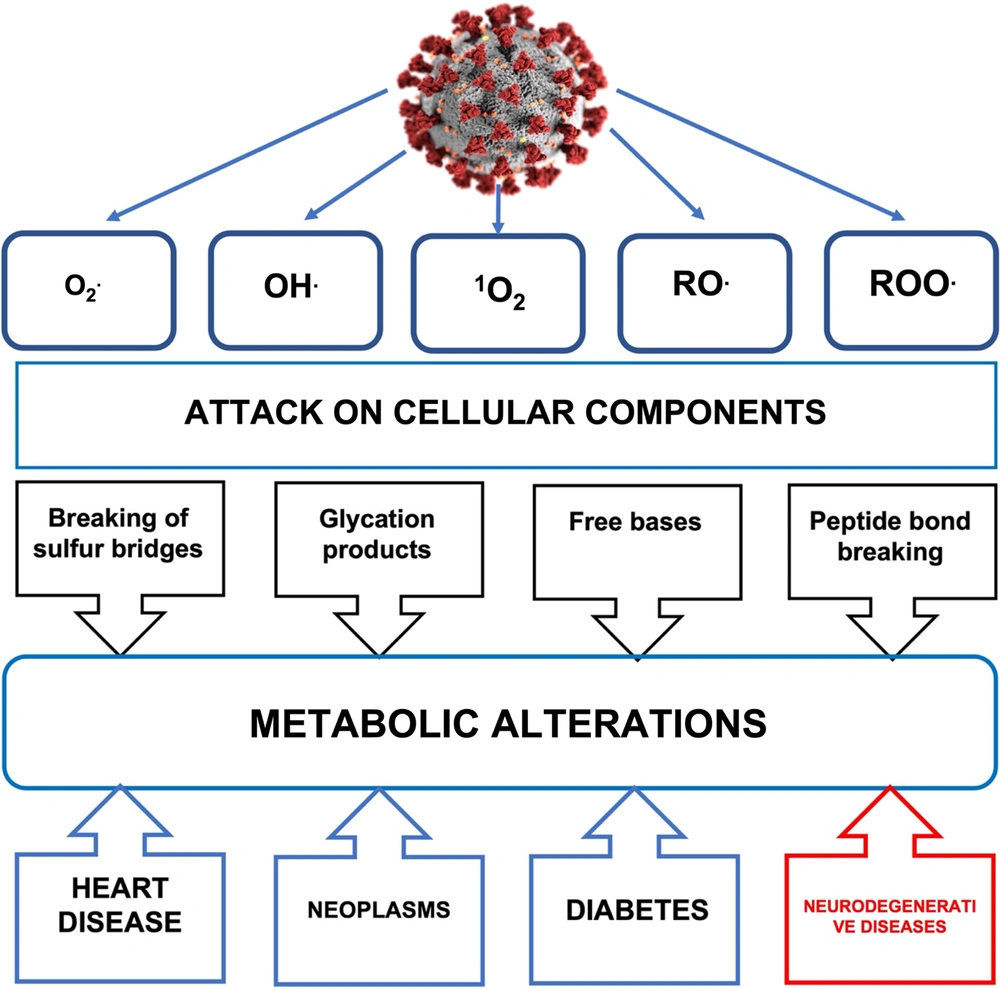
Dear Editor,
Oxygen (O2) is necessary for the life of most living beings, since it acts in mitochondrial respiration as the final acceptor of four electrons, giving rise to two water molecules and the formation of adenosine triphosphate (ATP). When O2 reduction is partial, reactive oxygen species (ROS) and free radicals are generated. O2 captures an electron, producing the radical anion superoxide (O2.-), which gives rise to hydrogen peroxide (H2O2) and to the hydroxyl radical (OH.) (1-4). ROS production occurs at the subcellular level in the mitochondria, lysosomes, peroxisomes, nuclear membrane, and in the cytoplasm of various cells, in which there are sources such as NADPH oxidases (NOX), a family of enzymes that use NADPH to reduce O2, producing O2.-. Oxidative stress is the imbalance between the generation of ROS and the antioxidant mechanisms in a biological system, where the former exceeds the capacity of the antioxidant defenses of said system (5, 6). ROS interact with molecular structures (DNA, proteins, lipids, and carbohydrates) leading to alterations in the activity of metabolic pathways and membranes, causing the accumulation of intracellular aggregates, mitochondrial dysfunction, excitotoxicity, and apoptosis. Oxidative stress is associated with molecular damage, which results in some pathologies (cancer, diabetes, and neurodegenerative diseases). Also, the increase in the production of free radicals promotes the massive entry of Ca2+ into the mitochondria, which causes the transition pore to form in the inner mitochondrial membrane (MTP), leading to a collapse in the proton electrochemical potential gradient, causing a decrease in ATP levels and an increase in ROS. Decreased ATP production results in depolarization of the plasma membrane and influx of Ca2+ through various ion channels, leading to loss of neuronal function and cell death. For the nervous system (NS), ROS participate in the maintenance of the physiological state since they regulate signaling pathways in processes of survival, development, plasticity, death, and inflammation (7-9). The NS is vulnerable to oxidative stress due to the high oxygen consumption required to maintain its high metabolic rate, with the consequent production of large amounts of ROS and a lower capacity of antioxidant mechanisms compared to other tissues. Oxidative stress is a fundamental factor in various processes of neuronal death, such as necrosis and apoptosis (10, 11). In necrosis, death results from loss of membrane integrity due to lipid peroxidation, oxidative damage to DNA and structural proteins (11). Apoptosis is characterized not only by loss of mitochondrial membrane potential and cytochrome C release, but also by protein peroxidation resulting in dysfunction of ATP synthase, dysfunction of electron transport chain complexes, and decreased concentration of antioxidant mechanisms such as glutathione (12, 13). When the body’s antioxidant defense capacity decreases due to the prooxidant action of a virus or bacteria, ROS increase, causing cellular and systemic damage, giving rise to oxidative stress itself. Like all RNA viruses, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has a cellular prooxidative action that activates the inflammatory response secondary to infection through this pathway, generating ROS that help perpetuate the damage caused by oxidative stress and loss of the antioxidant regulatory system. In coronavirus disease 2019 (COVID-19), the oxidative stress caused by the increase in ROS participates in the pathogenesis of the cytokine storm, in the coagulation mechanism, and the exacerbation of hypoxia (14). Elevated inflammatory and oxidative status can lead to mitochondrial oxidative stress and dysfunction, which can contribute to dysbiosis or imbalance in the balance of the gut microbiota, fueling the inflammatory and oxidative response (15). The relationship between viruses and ROS is due to the participation in three classes of viruses: (1) RNA viruses, (2) DNA viruses, and (3) retroviruses. Respiratory viruses, including SARS-CoV-2 (see Figure 1), induce the formation of ROS or free oxygen radicals in a deregulated manner. As a result of increased recruitment of inflammatory cells at the site of infection, viral infections alter antioxidant mechanisms, generating a prooxidant action, which leads to an unbalanced oxidative-antioxidant state and the consequent oxidative cell damage (16). SARS-Cov-2, by triggering the viral infection, causes the innate immune system (macrophages, dendrites, and monocytes) to detect the infection through pattern recognition receptors (PRRs), receptors that identify intrinsic molecules present in pathogens. Among the currently known PRRs, toll-like receptors (TLR) are mainly included, which activate a prooxidant response of macrophages, which leads to the activation of the TNF-α and of NADPH-oxidase in leukocytes, and which in turn mediate the production of ROS (17). In subjects with COVID-19, the hyperinflammatory state derived from the cytokine storm promotes iron dysregulation that manifests as hyperferritinemia associated with the severity of the disease and induces the production of ROS, and promotes oxidative stress (18). Nitric oxide synthase is an inducible enzyme in neutrophils, which, when activated, produces free oxygen radicals capable of combining with nitric oxide to give substances much more toxic than nitric oxide itself, such as peroxynitrite, thus increasing nitrosative stress. Neutrophilia generates an excess of ROS that exacerbates the host’s immunopathological response, resulting in more severe disease. The high proportion of neutrophils observed in critically ill patients with COVID-19 promotes a cascade of biological events that drives the pathological response and induces tissue damage, thrombosis, and red blood cell dysfunction, contributing to disease severity. The deleterious action of ROS on the functions of lung cells such as red blood cells can be seen as an important contributor to hypoxic respiratory failure, observed in the most severe cases of COVID-19, being its damaging effect on alveolar endothelial and epithelial cells, with procoagulative endotheliitis, and excess ROS (19). The smooth or rough endoplasmic reticulum (ER) is another generator of ROS. The ER oxidative stress activates proinflammatory signals and inflammasome formation, suggesting that ER stress exerts immunogenic effects and may be activated by excess lipids or proinflammatory cytokines (20-22).
In conclusion, COVID-19 represents an example of a viral infection associated with a cellular prooxidative action, infection, inflammation, coagulation with an increase in ROS, oxidative stress, and loss of the antioxidant system. It is essential to understand the role of free radicals in neurodegenerative processes to propose therapeutic objectives with the intention of delaying or preventing the progression of damage in neurodegenerative diseases due to COVID-19.