Abstract
Background:
Helicobacter pylori CagA oncoprotein was injected into mammalian host cells via type IV secretion system, where it was tyrosine phosphorylated at the Glu-Pro-Ile-Tyr-Ala (EPIYA) sequence. Tyrosine phosphorylation of CagA was shown to be a prerequisite for the induction of actin cytoskeletal rearrangement in AGS gastric epithelial cells. The needle-like projections, known as the hummingbird phenotype, are thought to be involved in gastric disease. Moreover, Pragmin, a mammalian protein, contains a single EPIYA motif, and tyrosine phosphorylation of Pragmin at EPIYA motif in AGS cells induce cell-morphological changes, which are characterized by elongated cell shape with invasive phenotype that contributes to tumor invasion and metastasis.Methods:
In this study, AGS cells were transfected by CagA or/and Pragmin using lipofectamine 2000 reagent, then, cell-morphological changes were investigated using light microscope. Finally, elongated cells were counted and the results were compared.Results:
Our results revealed that there is a competition between CagA and Pragmin to interact with CSK via EPIYA motif. We also found that although the cell-morphological changes are particularly dependent on tyrosine phosphorylation at EPIYA motifs in both, changes of cell morphology are different in CagA and Pragmin transfected cells.Conclusions:
Our findings suggest that the mechanisms inducing the elongated cell morphology in CagA or Pragmin transfected cells may be different.Keywords
Pragmin Morphological Changes EPIYA Motif AGS Cells CagA Helicobacter pylori
1. Background
Helicobacter pylori was recognized as a type I carcinogen, and chronic infection with H. pylori has been linked to atrophic gastritis, peptic ulcers, and gastric cancer (1). H. pylori strains are frequently segregated into cagA-positive and cagA-negative strains. Compared with cagA- H. pylori strains, cagA+ strains significantly increase the risk of developing severe gastritis and gastric carcinoma (2, 3). The cagA gene, which is located at cag pathogenicity island (cagPAI) in the H. pylori chromosome, encodes a 120 to 145-kDa immunodominant protein, CagA (4, 5). CagA oncoprotein is delivered directly by the bacterium into gastric epithelial cells via type IV secretion system and localized to the inner side of the plasma membrane. Tyrosine phosphorylation of CagA occurs by Src family kinases (SFKs) such as c-Src, Lyn, Fyn, and Yes or c-Abl kinase at the Glu-Pro-Ile-Tyr-Ala (EPIYA) sequence, which is present in variable numbers in its C-terminal region (6-8). Depending on the geographic region, 4 distinct EPIYA sites have been described including EPIYA-A, -B, -C, and -D, each of which is conserved (9). Remarkably, the EPIYA-A and EPIYA-B motif are found in strains throughout the world, but EPIYA-C is mainly present in strains from Asian countries, Europe, and North America (Western type), while the EPIYA-D motif predominates in the far East such as China, Japan, and Korea (East Asian type) (9). By tyrosine phosphorylation at EPIYA motifs, CagA is enabled to interact with huge numbers of SH2 domains containing proteins to disturb host cell signal transduction for better colonization and spread of bacterial infection. In this regard, interaction of EPIYA motifs of CagA (EPIYA-A or EPIYA-B) with CSK (C-terminal Src kinase), (EPIYA-C or EPIYA-D) with SHP2 (SH2 domain-containing protein tyrosine phosphatase 2), (EPIYA-B) with PI3K (phosphatidylinositol 3-kinase), and Crk were demonstrated (10-15). However, the EPIYA segment (s) responsible for the Crk interaction is not known. Furthermore, it was shown that H. pylori induced a characteristic morphology of host epithelial cells, which has been referred to as the hummingbird phenotype; this was resulting from regulation of both the actin cytoskeleton and focal adhesion and it may be involved in carcinogenesis. Notably, it has been emphasized by many findings indicating that elongation morphology of host cell is strictly dependent on CagA injection (16-18). Despite the discovery of many target molecules in host cells, which is caused by H. pylori infection, the related processes that lead to hummingbird phenotype are still unclear.
From the mammalian side, it was reported that Pragmin contains a functional EPIYA motif at N-terminus. After tyrosine phosphorylation at EPIYA motif, Pragmin can interact with CSK. Interestingly, it was found that the cells with high expression of Pragmin have the elongated morphology that contributes to tumor invasion and metastasis (19-21). In this regard, it was shown that ectopic expression of Pragmin in human pancreatic duct epithelial (PDAC) cells induced an elongated mesenchymal-like cell morphology, which is associated with increased cell migration and invasion (22). Taken to gather, we selected CagA and Pragmin for further studying because both contain EPIYA motifs, showed elongated cell morphology after tyrosine phosphorylation, and both can interact with CSK, a common target protein which contains SH2 domain.
2. Objectives
The present study was conducted to obtain more information about tyrosine phosphorylation at EPIYA motif dependent mechanisms in the cells, and particularly their potential role in cell invasion and carcinogenesis, which can be used as therapeutic strategies.
3. Methods
3.1. Expression Vectors
A pCMV-based mammalian expression vector for rat Pragmin was kindly provided by Dr. M. Negishi, (Kyoto University, Japan). Wild-type CagA (CagA-ABCCC derived from H. pylori NCTC11637 Western strain) was cloned into the pSP65Srα mammalian expression vector; pSP65Srα mammalian expression vector was also used as a control (empty vector).
3.2. Cell Culture and Transfection
AGS human gastric epithelial cells were cultured in RPMI 1640 medium supplemented, with 10% fetal bovine serum (FBS), at 37°C, under a 5% CO2 humidified atmosphere; 10 μg expression vectors were totally transfected into 4.5 × 105 AGS cells in a 60-mm dish using lipofectamine 2000 (Invitrogen), according to the manufacturer’s instructions. Cell morphology was examined under light microscopy (Nikon, Tokyo, Japan) 20 hours after transfection. We used pSP65Srα (empty vector) as a control. Elongated cells were counted in random fields of surface cell culture dish in groups of cells, which were transfected with pSP65Srα, CagA, Pragmin, and CagA/Pragmin.
3.3. Statistical Analysis
Statistical analyses were performed using Student’s t test and the MedCalc software (Version 12.1.4) was used.
4. Results
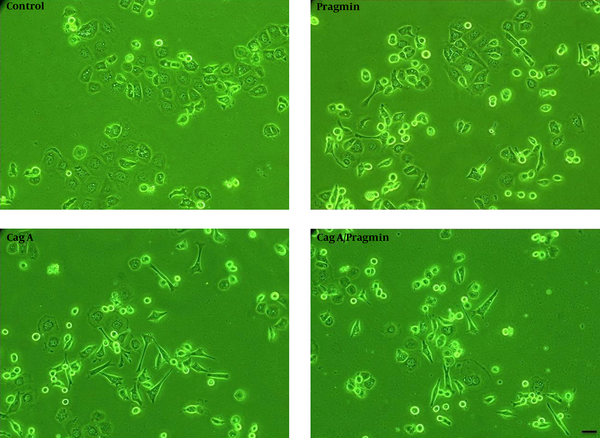
To investigate cell morphology, AGS cells were cultured one day before transfection using lipofectamine 2000, they were then transiently transfected with pSP65Srα (empty vector), Pragmin, CagA, or CagA/Pragmin vectors. On the other hand, we transfected AGS cells by 10 μg pSP65Srα, 5 μg Pragmin + 5 μg pSP65Srα, 5 μg CagA + 5 μg pSP65Srα, and 5 μg CagA + 5 μg Pragmin. Cell morphological changes were evaluated 24 hours after transfection using light microscope, then, elongated cells were counted randomly in different fields in each of the dishes (Figure 1). Our results revealed that cell morphology is hummingbird phenotype in CagA-transfected cells, whereas Pragmin-transfected cells showed elongated morphology (or invasive form). Next, we counted the number of cells with elongated morphology and compared them (Figure 2). Our results showed that the difference between Pragmin-transfected cells and empty vector transfected cells was significant. Moreover, the difference between elongated cells obtained from CagA-transfected cells and empty vector transfected cells was also significant (Figure 2). Importantly, our results demonstrated no significant difference between CagA-transfected cells and CagA/Pragmin transfected cells. Percentage of elongated cells was 6.6%, 35.1%, 62.3%, and 63.9% in control (pSP65Srα), Pragmin, CagA, and CagA/Pragmin transfected cells, respectively (Figure 2).
Morphological Investigation of AGS cells by Using Microscopy
Percentage of Elongated Cells Was Shown

In fact, the presence of common step (interaction CagA or Pragmin with CSK via EPIYA motif) and competition between CagA and Pragmin were thought to influence the number of elongated cells. Also, downstream related molecules in induction of cells with elongated morphology were different. Taken together, our results suggest that in spite of some functional homology effects between CagA oncoprotein and Pragmin in AGS cells to induce cell-morphological changes, the changes in cell morphology are different, and thus it seems that the related mechanisms involved in these processes may also be different.
5. Discussion
In the present study, we compared morphological changes of AGS cells between CagA-transfected cells and Pragmin-transfected cells. Both were tyrosine phosphorylated at EPIYA motif and were shown to influence cell morphology. CagA is a bacterial effector protein containing EPIYA motif, whereas Pragmin is a mammalian protein containing EPIYA motif. We found that the morphology of AGS is elongated shape or has an invasive form in Pragmin-transfected cells, but the morphology of AGS cells is polygonal shape and needle form in CagA-transfected cells (or hummingbird phenotype). Our results revealed that the number of elongated cells in CagA transfected cells in the presence or absence of Pragmin is the same. Consistent with our results, Safari et al. reported that in the presence of CagA H. pylori, the amount of CSK /Pragmin interaction was reduced. In fact, they found the competition between CagA and Pragmin to interact with CSK in tyrosine phosphorylation manner at EPIYA motif (19). Of note, it was demonstrated that CagA/CSK interaction occurs at plasma membrane, whereas Pragmin interact with CSK at cytoplasm, and thereby the possible mechanisms that are involved in related downstream molecules to induce elongated cells may also be different. In this regard, it was reported that Src-mediated CagA phosphorylation is followed by a rapid inactivation of Src kinase activity by the binding of CagA to Csk, and then, Src kinase inactivation leads to the dephosphorylation of Src target proteins such as vinculin, ezrin, and cortactin, which are important in the process of regulation of the actin cytoskeleton and focal adhesions. This process contributes to inducing morphological changes of H. pylori-infected cells (18, 23, 24). Also, it was shown that phosphorylation of cortactin (serine 405) was mediated by ERK1/2 kinases, which might trap activated FAK, leading to a disturbed turnover of focal adhesions and cell elongation morphology (25). In another study, the activation of SHP-2 phosphatase activity was reported to inactivate FAK (focal adhesion kinase) in cells that ectopically express CagA (26). Inactivated FAK cannot be localized in focal adhesions and might support the development of the elongated cell phenotype. Of note, after inactivation of Src, CagA phosphorylation is sustained by Abl kinase. However, CagA dependent downstream effects in this way have not yet been known (7, 8).
Recently, it was found that CSK/Pragmin interaction stimulates kinase activity of CSK, which is essential to phosphorylate Pragmin on Y238, Y343, and Y391 (The latter is located at EPIYA motif.). Moreover, interaction Pragmin with vinculin, a focal adhesion component(s), was found. Therefore, it seems that vinculin recruits Pragmin and Pragmin- associated CSK to focal adhesion spots to elevate cell motility and elongated cells formation, demonstrating the major role of CSK/Pragmin interaction in inducing invasive shape of the cell in MKN7 human gastric epithelial cells (20). Further studies should be conducted to better understand tyrosine phosphorylation at EPIYA motif dependent mechanisms, which involve in carcinogenesis, to design anticancer drugs.
Acknowledgements
References
-
1.
Parkin DM, Bray F, Ferlay J, Pisani P. Global Cancer Statistics, 2002. CA Cancer J Clin. 2005;55(2):74-108. https://doi.org/10.3322/canjclin.55.2.74.
-
2.
Kuipers EJ, Perez-Perez GI, Meuwissen SG, Blaser MJ. Helicobacter pylori and atrophic gastritis: importance of the cagA status. J Natl Cancer Inst. 1995;87(23):1777-80. [PubMed ID: 7473834]. https://doi.org/10.1093/jnci/87.23.1777.
-
3.
Peek RJ, Miller GG, Tham KT, Perez-Perez GI, Zhao X, Atherton JC, et al. Heightened inflammatory response and cytokine expression in vivo to cagA+ Helicobacter pylori strains. Lab Invest. 1995;73(6):760-70. [PubMed ID: 8558837].
-
4.
Covacci A, Censini S, Bugnoli M, Petracca R, Burroni D, Macchia G, et al. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci U S A. 1993;90(12):5791-5. [PubMed ID: 8516329]. https://doi.org/10.1073/pnas.90.12.5791.
-
5.
Tomb JF, White O, Kerlavage AR, Clayton RA, Sutton GG, Fleischmann RD, et al. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388(6642):539-47. [PubMed ID: 9252185]. https://doi.org/10.1038/41483.
-
6.
Covacci A, Rappuoli R. Tyrosine-phosphorylated bacterial proteins: Trojan horses for the host cell. J Exp Med. 2000;191(4):587-92. [PubMed ID: 10684850]. https://doi.org/10.1084/jem.191.4.587.
-
7.
Poppe M, Feller SM, Romer G, Wessler S. Phosphorylation of Helicobacter pylori CagA by c-Abl leads to cell motility. Oncogene. 2007;26(24):3462-72. [PubMed ID: 17160020]. https://doi.org/10.1038/sj.onc.1210139.
-
8.
Tammer I, Brandt S, Hartig R, Konig W, Backert S. Activation of Abl by Helicobacter pylori: a novel kinase for CagA and crucial mediator of host cell scattering. Gastroenterology. 2007;132(4):1309-19. [PubMed ID: 17408661]. https://doi.org/10.1053/j.gastro.2007.01.050.
-
9.
Hatakeyama M. Oncogenic mechanisms of the Helicobacter pylori CagA protein. Nat Rev Cancer. 2004;4(9):688-94. [PubMed ID: 15343275]. https://doi.org/10.1038/nrc1433.
-
10.
Backert S, Tegtmeyer N, Selbach M. The versatility of Helicobacter pylori CagA effector protein functions: The master key hypothesis. Helicobacter. 2010;15(3):163-76. [PubMed ID: 20557357]. https://doi.org/10.1111/j.1523-5378.2010.00759.x.
-
11.
Tsutsumi R, Higashi H, Higuchi M, Okada M, Hatakeyama M. Attenuation of Helicobacter pylori CagA x SHP-2 signaling by interaction between CagA and C-terminal Src kinase. J Biol Chem. 2003;278(6):3664-70. [PubMed ID: 12446738]. https://doi.org/10.1074/jbc.M208155200.
-
12.
Higashi H, Tsutsumi R, Muto S, Sugiyama T, Azuma T, Asaka M, et al. SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science. 2002;295(5555):683-6. [PubMed ID: 11743164]. https://doi.org/10.1126/science.1067147.
-
13.
Higashi H, Tsutsumi R, Fujita A, Yamazaki S, Asaka M, Azuma T, et al. Biological activity of the Helicobacter pylori virulence factor CagA is determined by variation in the tyrosine phosphorylation sites. Proc Natl Acad Sci U S A. 2002;99(22):14428-33. [PubMed ID: 12391297]. https://doi.org/10.1073/pnas.222375399.
-
14.
Selbach M, Paul FE, Brandt S, Guye P, Daumke O, Backert S, et al. Host cell interactome of tyrosine-phosphorylated bacterial proteins. Cell Host Microbe. 2009;5(4):397-403. [PubMed ID: 19380118]. https://doi.org/10.1016/j.chom.2009.03.004.
-
15.
Suzuki M, Mimuro H, Suzuki T, Park M, Yamamoto T, Sasakawa C. Interaction of CagA with Crk plays an important role in Helicobacter pylori-induced loss of gastric epithelial cell adhesion. J Exp Med. 2005;202(9):1235-47. [PubMed ID: 16275761]. https://doi.org/10.1084/jem.20051027.
-
16.
Bourzac KM, Botham CM, Guillemin K. Helicobacter pylori CagA induces AGS cell elongation through a cell retraction defect that is independent of Cdc42, Rac1, and Arp2/3. Infect Immun. 2007;75(3):1203-13. [PubMed ID: 17194805]. https://doi.org/10.1128/IAI.01702-06.
-
17.
Segal ED, Cha J, Lo J, Falkow S, Tompkins LS. Altered states: involvement of phosphorylated CagA in the induction of host cellular growth changes by Helicobacter pylori. Proc Natl Acad Sci U S A. 1999;96(25):14559-64. [PubMed ID: 10588744]. https://doi.org/10.1073/pnas.96.25.14559.
-
18.
Selbach M, Moese S, Hurwitz R, Hauck CR, Meyer TF, Backert S. The Helicobacter pylori CagA protein induces cortactin dephosphorylation and actin rearrangement by c-Src inactivation. EMBO J. 2003;22(3):515-28. [PubMed ID: 12554652]. https://doi.org/10.1093/emboj/cdg050.
-
19.
Safari F, Murata-Kamiya N, Saito Y, Hatakeyama M. Mammalian Pragmin regulates Src family kinases via the Glu-Pro-Ile-Tyr-Ala (EPIYA) motif that is exploited by bacterial effectors. Proc Natl Acad Sci U S A. 2011;108(36):14938-43. [PubMed ID: 21873224]. https://doi.org/10.1073/pnas.1107740108.
-
20.
Senda Y, Murata-Kamiya N, Hatakeyama M. C-terminal Src kinase-mediated EPIYA phosphorylation of Pragmin creates a feed-forward C-terminal Src kinase activation loop that promotes cell motility. Cancer Sci. 2016;107(7):972-80. [PubMed ID: 27116701]. https://doi.org/10.1111/cas.12962.
-
21.
Leroy C, Fialin C, Sirvent A, Simon V, Urbach S, Poncet J, et al. Quantitative phosphoproteomics reveals a cluster of tyrosine kinases that mediates SRC invasive activity in advanced colon carcinoma cells. Cancer Res. 2009;69(6):2279-86. [PubMed ID: 19276381]. https://doi.org/10.1158/0008-5472.CAN-08-2354.
-
22.
Tactacan CM, Phua YW, Liu L, Zhang L, Humphrey ES, Cowley M, et al. The pseudokinase SgK223 promotes invasion of pancreatic ductal epithelial cells through JAK1/Stat3 signaling. Mol Cancer. 2015;14:139. [PubMed ID: 26215634]. https://doi.org/10.1186/s12943-015-0412-3.
-
23.
Selbach M, Moese S, Backert S, Jungblut PR, Meyer TF. The Helicobacter pylori CagA protein induces tyrosine dephosphorylation of ezrin. Proteomics. 2004;4(10):2961-8. [PubMed ID: 15378755]. https://doi.org/10.1002/pmic.200400915.
-
24.
Moese S, Selbach M, Brinkmann V, Karlas A, Haimovich B, Backert S, et al. The Helicobacter pylori CagA protein disrupts matrix adhesion of gastric epithelial cells by dephosphorylation of vinculin. Cell Microbiol. 2007;9(5):1148-61. [PubMed ID: 17217431]. https://doi.org/10.1111/j.1462-5822.2006.00856.x.
-
25.
Tegtmeyer N, Wittelsberger R, Hartig R, Wessler S, Martinez-Quiles N, Backert S. Serine phosphorylation of cortactin controls focal adhesion kinase activity and cell scattering induced by Helicobacter pylori. Cell Host Microbe. 2011;9(6):520-31. [PubMed ID: 21669400]. https://doi.org/10.1016/j.chom.2011.05.007.
-
26.
Tsutsumi R, Takahashi A, Azuma T, Higashi H, Hatakeyama M. Focal adhesion kinase is a substrate and downstream effector of SHP-2 complexed with Helicobacter pylori CagA. Mol Cell Biol. 2006;26(1):261-76. [PubMed ID: 16354697]. https://doi.org/10.1128/MCB.26.1.261-276.2006.