1. Background
Screening mammography is widely accepted as an effective and safe imaging modality to detect breast cancer in its primary stages (1). A focal asymmetry not present on prior mammograms or increased in size or conspicuity is defined as developing asymmetry (2). In contrast to mass, asymmetry has interspersed fat with absent convex margins (2, 3). This entity has been reported to be seen on 0.16% of screening and 0.11% of diagnostic mammograms (4, 5). Because developing asymmetries are uncommon, few studies are available in the literature (3-5).
Based on the literature, developing asymmetry is associated with malignancy in 12.8% of screening-detected and 26.7% of diagnostic imaging-detected cases (4). All developing asymmetries are recommended to be biopsied, unless they can be explained clearly as a benign entity, such as hematoma, weight loss, fibrocystic change, or hormonally stimulated fibroglandular tissue (6, 7).
Despite the increasing use of ultrasound in the evaluation of breast lesions, the likelihood of finding the US correlate for a developing asymmetry remains unknown (8). Breast magnetic resonance imaging (MRI) has an evolving role in breast imaging and has the potential for use in further evaluation of developing asymmetries.
2. Objectives
The current study aimed at evaluating the frequency of developing asymmetry in the opportunist screening of a population from the Middle East, with US and MRI imaging findings, and the histopathologic correlations of developing asymmetry.
3. Methods
3.1. Study Population and Data Collection
This retrospective observational study was approved by our university review board, which waived the requirement for written informed consent. This was a cross-sectional study on a database of opportunistic screening mammography at a university-affiliated main academic medical center, cancer center, breast clinic, from January 1, 2017, to December 31, 2018.
Non diagnostic Mammograms (n = 12,169) were evaluated for developing asymmetries. All mammographies were taken, using a Hologic Selenia digital mammography machine. Patients with any clinical concerns, such as mass, pain, or nipple discharge, and patients under chemotherapy or hormone replacement therapy were excluded from the study. Developing asymmetry was defined as a focal asymmetry not present on prior mammograms or increased in size or conspicuity.
Developing asymmetry was reported in 54 patients. Two breast radiologists with 20 and 8 years of breast imaging experience re-evaluated those 54 cases and confirmed the existence of developing asymmetry. Following the detection of developing asymmetry, additional mammographic views and/or the targeted US were performed, and all findings were recorded. Demographic data, including age, previous medical history, and family history of breast cancer were collected. Mammographic breast composition, presence of calcification and architectural distortion in mammography, and US and MRI findings were recorded. The patients who did not perform biopsy were followed by mammography, US, or MRI for at least 1 year.
3.2. Statistical Analysis
Descriptive statistics were reported as mean ± SD for normally distributed continuous data, median (quartile 1 - quartile 3) for skewed continuous variables, and frequency (percent) for categorical variables. The independent samples t test, Mann-Whitney U test, and chi-square test with exact P-value were applied to evaluate the variables. Normality assumption was tested, using the Shapiro-Wilk test, which is powerful for small sample size studies. All statistical analyses were performed by SPSS V.25.
4. Results
4.1. Demographic Findings
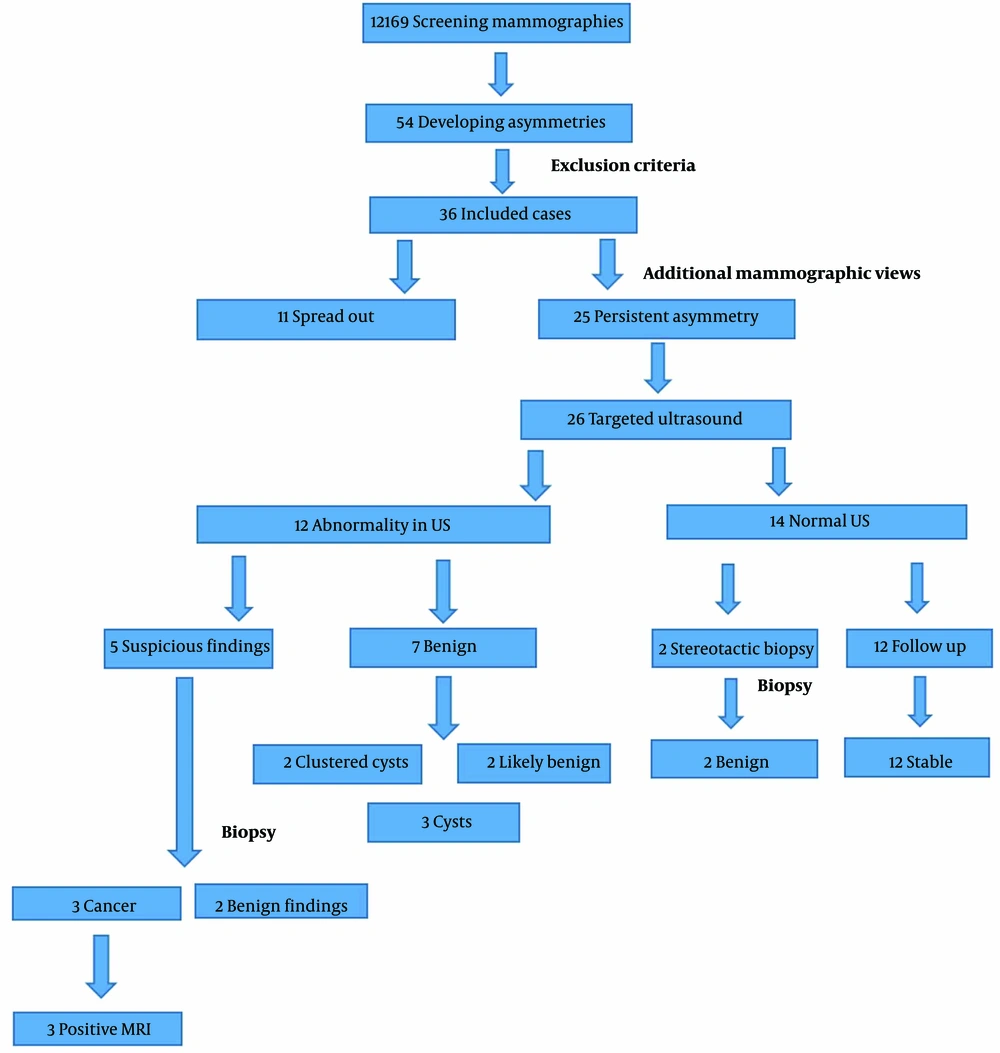
Of the 12 169 women, who were referred to our center for screening mammography, 54 (0.44%) had developed asymmetry. From these, 36 patients fulfilled the inclusion criteria and were included in the final analyses. The mean age of participants was 60 ± 8.4 years. Positive family history of breast cancer was found in 7 (19.4%) patients. A t test analysis showed a significant association between positive family history and breast cancer in these patients (P = 0.04).
4.2. Mammographic Findings
Among the 36 identified developing asymmetries, 21 lesions (58.3%) were in the right breast, and the upper outer was the most involved quadrant (27.8% of lesions). Seventeen (47.2%) lesions were enlarged focal asymmetries compared to the previous mammography, and the rest were new (52.7%).
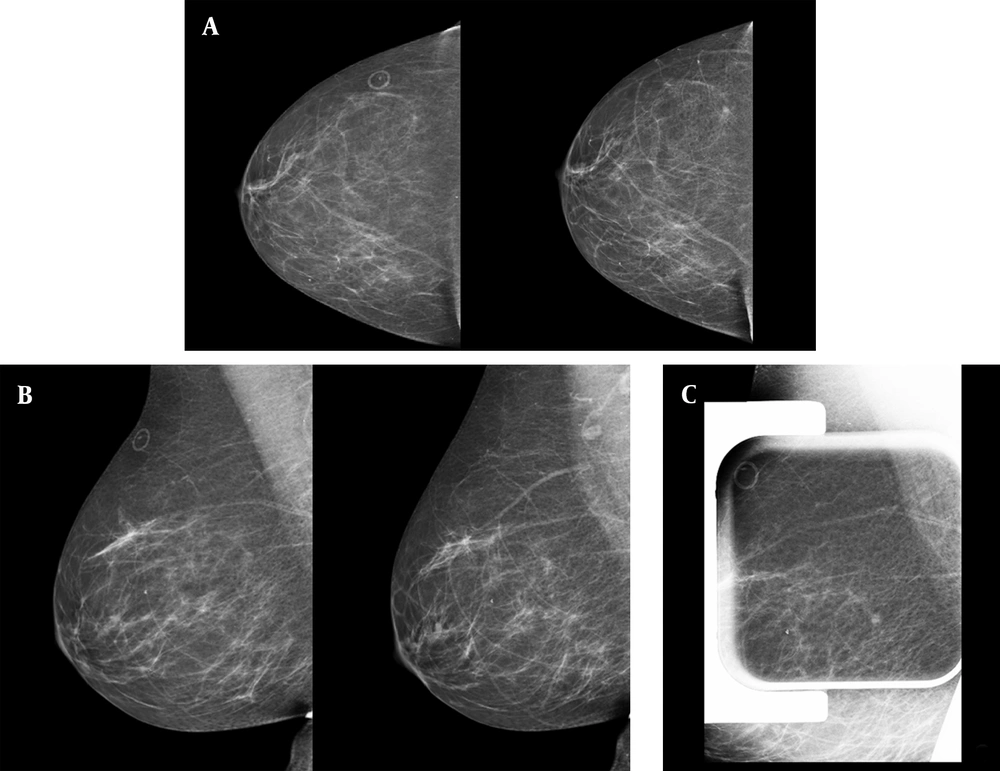
Type C breast composition (heterogeneous dense breast) was reported more commonly (15 patients; 42.9%). The commonest concomitant finding in mammography was benign type microcalcifications (14 patients; 40.0%). Additional mammographic views, including compression spot view, demonstrate spread out of the developing asymmetry in 11 (30.5%) cases. One-year follow-up of these patients also showed them unchanged. The patients with persistent asymmetries all received an ultrasound. Two patients with persistent asymmetries and normal ultrasound underwent stereotactic biopsy because of shape and margin (Figure 1). The histopathologic findings were benign in both of them.
Screening mammography of a 56-year-old woman demonstrates a tiny developing focal asymmetry in the posterior upper outer part of the right breast. A, craniocaudal views of two consecutive years; the marker shows a skin mole; B, mediolateral oblique views of the same two consecutive years; C, focal compression spot view in mediolateral oblique view projection shows persistent asymmetry. In the targeted ultrasound, no abnormality was detected; the stereotactic biopsy of the focal asymmetry had benign pathology results of hyperplasia and stromal fibrosis.
4.3. Ultrasound Findings
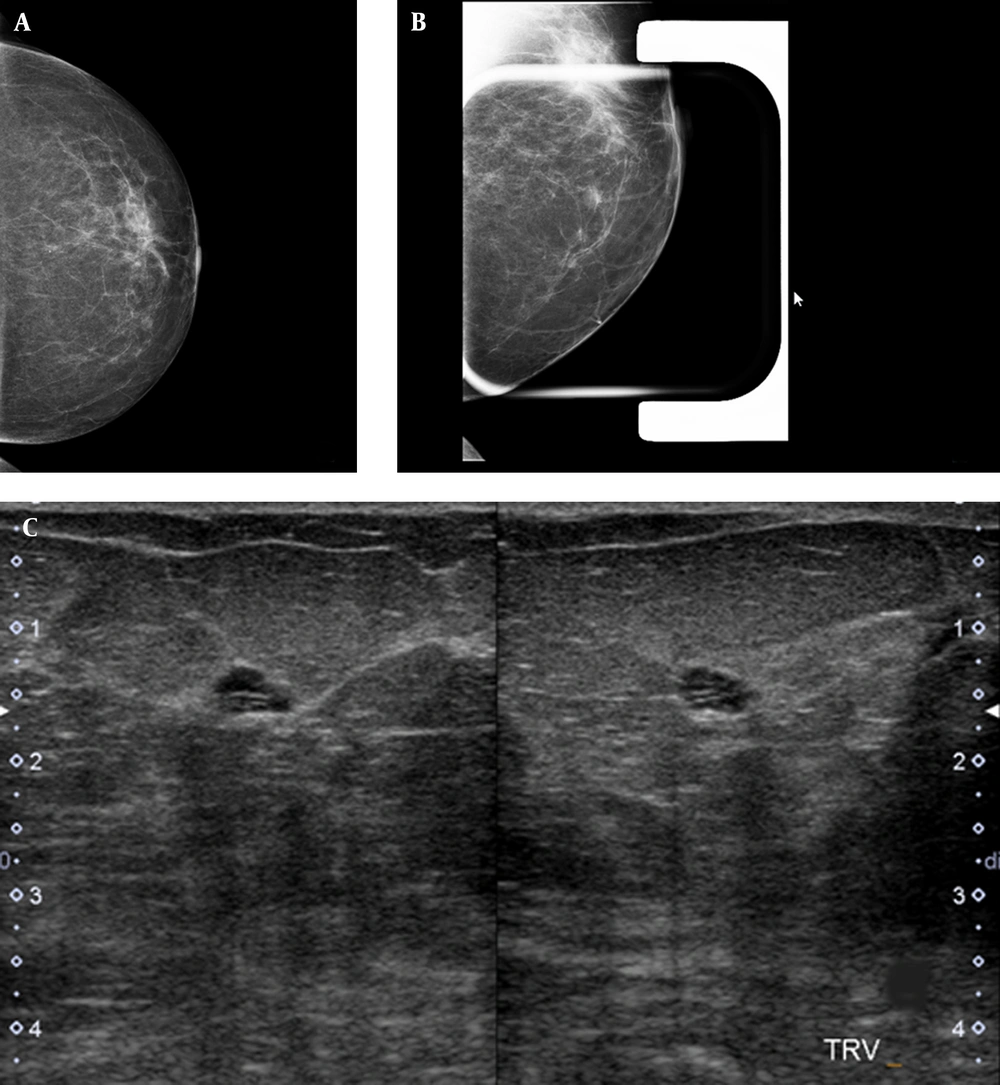
Twenty-six patients (72.2%) underwent ultrasonography (Esaote MyLab 50 Ultrasound System; multifrequency probe), from whom 14 (38.8%) patients had no correlated findings, 3 patients (8.3%) had a cyst lesion at the site of developing asymmetry (Figure 2), 2 patients (5.5%) had probably benign tumor at the US, and 2 patients (5.5%) had clustered cyst-fibrocystic patch with no malignant changes in follow-up imaging (both 6-months and 1-year follow up imaging). In 2 patients (5.5%), a suspicious non-mass hypoechoic area was seen at the site of developing asymmetry, which was correlated with invasive ductal carcinoma in histopathologic evaluation. In 3 patients (8.3%), a suspicious mass was seen, 1 of whom had invasive ductal carcinoma in histopathologic evaluation. Three of 5 patients with suspicious ultrasound findings were malignant in histopathologic evaluation. Fisher’s exact probability test revealed a significant association between sonographic findings and the risk of malignancy in patients having developing asymmetry (P = 0.003).
A single view developing asymmetry is seen in the inner part of the left breast. A, left breast craniocaudal view; B, a focal compression spot view of the left breast demonstrates an oval circumscribed mass; C, the targeted ultrasound of the same area, corresponding to the mammographic finding, shows a thin clustered cyst.
4.4. MRI Findings
A breast MRI was done on 3 patients (8.3%). Findings included segmental clumped non-mass enhancement with curve 2 kinetic (BIRADS = 4, suspicious finding) in 1 patient and mass with rim enhancement in the other 2 patients. All of the patients had malignant findings in histopathologic evaluation.
4.5. Histopathologic Findings
A biopsy was performed in 7 patients (19.4%). A stereotactic biopsy was performed in 2 patients (5.5%) and a US-guided biopsy in 5 patients (13.8 %). Malignant findings compatible with invasive ductal carcinoma were present in 3 patients (8.3% of all patients). Ultrasound and MRI correlations were found in all of the malignant cases. Other patients who had undergone breast biopsy had benign histopathological findings, including adenosis, fibrocystic changes, and benign ductal hyperplasia. The positive predictive value (PPV) of developing asymmetry for malignancy was 8.3%. Figure 3 shows the frequency of developing asymmetry in our patients and how we approached them.
5. Discussion
Diagnosis of developing asymmetry can be challenging. Firstly, because it is similar to normal fibroglandular; secondly, sometimes, it is difficult to determine whether a focal asymmetry or asymmetry is developing slowly or appears more conspicuous because of technical differences, such as compression or positioning (6). On the other hand, although the change in imaging appearance raises the possibility of malignancy, it is nonspecific, because many benign lesions also demonstrate change (9). However, malignant lesions might also result in developing asymmetry; so, it is important to know how to approach this mammographic finding to not miss malignant lesions and also prevent unnecessary biopsy (10).
The prevalence of developing asymmetry was 0.44% in our study, which is higher than reported by Leung and Sickles (0.16% prevalence of developing asymmetry in women referred for screening mammograms) (5). Among 36 patients with developing asymmetries and whose data were analyzed, 3 patients were proven to have cancer. The PPV of developing asymmetry for malignancy was 8.3%. These findings emphasize the importance of further work-up in developing asymmetries. We propose to consider developing asymmetry as BIRADS 4 and to biopsy them unless the presence of summation artifact in additional mammographic view or definitive benign correlations (e.g., cyst in ultrasound) is confirmed.
The US is a safe and low-cost diagnostic tool in breast imaging; however, its role in developing asymmetry is less well established. In a study by Shetty and Watson (8) on 20 patients with developing asymmetry, 28% of patients, including 2 of 7 cancers, had no US correlation. In the Chesebro et al.’s study, the presence of US or MRI findings was predictive of malignancy in developing asymmetries with borderline mammographic significance (6). A recent study by Giess et al. reported non-mass findings as to the most common US corresponding lesions for developing asymmetries; moreover, these authors showed that most malignant developing asymmetries present as a hypoechoic mass in the US (11).
In our study, suspicious findings were detected in the US of 5 patients. Two patients had non-mass hypoechoic findings, 3 patients had mass who underwent biopsy, and 3 of them had invasive ductal carcinoma. Our results show that when a corresponding US finding is found, the chance of a developing asymmetry being malignant increases significantly (P = 0.003). Although in our study all of the patients with malignancy had US correlates, other previous studies showed that developing asymmetry could be malignant, even without a US-correlated lesion (5, 6).
Occasionally, MRI can be used as a problem-solving modality in evaluating developing asymmetry, especially when the US is not helpful (12). In the Chesebro et al.’s study, breast MRI showed a negative predictive value of 100% and a false-negative rate of 0% (6). Moreover, in that study, all 10 of the developing malignant asymmetries had an MRI correlation (PPV, 100%). In our study, MRI correlates were found in all 3 patients with invasive ductal carcinoma. Although contrast-enhanced MRI has a high sensitivity in detecting cancer (13), it has high false-positive results resulting in unnecessary biopsy (14). Hence, it is suggested to consider MRI only when other diagnostic modalities have failed to determine the diagnosis (7, 12).
Our results also showed the association between a family history of breast cancer and the risk of malignancy. Hence, family history in patients with developing asymmetry should be considered when evaluating the risk of malignancy.
Our results should be interpreted considering the limitations of sample volume, which was small; considering the low prevalence of developing asymmetry, it would be better to perform similar studies in a longer period and with a larger sample size.
To sum up, our results show that most of the developing asymmetries are due to summation artifacts. So, using additional mammographic views should be considered the first step in this situation. Using the US is another helpful modality, MRI could be used when these techniques had not yielded a reasonable etiology for developing asymmetry.