1. Background
Growth is one of the major challenges in premature and low birth weight neonates (1). Premature infants should increase energy and nutrients intake for their rapid growth needs to achieve the optimal growth (2). In order to achieve optimal growth in these infants, the intrauterine growth process should be continued outside the uterus environment up to 40 weeks after fertilization, reaching the normal growth and accumulation of nutrients in the post discharge period (3). Evidence suggests that premature infants are inadequately swallowed by the mother’s vaginal flora because of the rapid passage of the delivery channel, thus initial colonization is inadequate with a low diversity of bacteria. Neonates also have immature immune defenses, resulting in an increase inflammatory responses in the gut lumen (4). For this reason, the full feeding in these infants is delayed, as a result, these infants develop extrauterine growth restriction (EUGR) (5). Infants with EUGR are at high risk of developing the longterm complications, including ischemic heart disease, abnormal glucose tolerance testing, type 2 diabetes mellitus and hypertension, to prevent these complications and reduce the severity, there is a need for strategies to shorten the time to reach full feeding (5, 6).
Premature infants have implantable physiological systems, inadequate development and increased gut permeability. Consequently, pathogenic bacteria passing through the gut lumen which may cause systemic infections (7). Gut micro-flora plays an important role in the development of the sensory-motor activity of the gut through the release of bacterial agents, fermentation products, gut neuroendocrine factors, and mediators released by the intestinal immune system (4). Necrotizing enterocolitis (NEC) is one of the common causes of death in preterm infants (8), prematurity and abnormal colonization of bacteria play a major role in the development of NEC. Probiotics administration reduces the risk of NEC and mortality in preterm infants (9), using probiotics could improve feeding tolerance leading to better growth and decreases the incidence of NEC in premature infants (3). Probiotic supplementation could result in higher amounts of lactobacillus and bifidobacterium in gut lumen and influence on feeding in preterm infants (10).
Probiotics are live microbial supplements that colonize the gut, with specific properties for gelling intestinal epithelium, and potentially exert health benefit to the host (11, 12). The proposed mechanisms by probiotics to improve feeding tolerance include gut balance shifting from a potentially harmful micro-flora to useful types, enhancing intestinal mucus barrier function, preventing bacteria colonization or their products and modifying host responses to these microbial products (13). Also, probiotics enhance the innate immune defense of premature infants by increasing the production of mucosal IgA, enhancing leukocyte phagocytosis, and reducing the production of inflammatory cytokines (14). Although many developed countries are already using probiotics routinely in preterm neonates for prevention of NEC (15), the specific mechanism of probiotic supplementation on gastrointestinal function is not yet clear (16), especially in very low birth weight infants (14). In this framework studies advocated that the administration of probiotics reduced feeding intolerance, but the matter is still controversial. This study was designed to investigate the time to reach full intestinal feeds and intestinal feeding tolerance in neonates 1000 to 2500 grams.
2. Objectives
We hypothesized that by establishing a normal intestinal flora probiotic could reduce the incidence of feed intolerance. Therefore we examined the effect of probiotics on time to reach full enteral feeds (120 cc/kg/day) in premature newborns.
3. Methods
This study was a double-blind randomized clinical trial. The present study was registered at the Iranian Center for Clinical Trials (IRCT Code = 201706129075N2), and has been approved by the Ethics Committee of Guilan University of Medical Sciences (Ethical Code: IR.GUMS.REC.1396.302). Study details were given to the mothers and a written consent was obtained from the parents of the infants for participation in the study.
The samples were premature infants with gestational age of less than 36 weeks and birth weight of 1000 - 2500 gr, admitted to the neonatal intensive care unit (NICU) of 17th Shahrivar Children’s hospital in Rasht, Iran. In this study, 58 preterm infants were randomly assigned into two groups of 29 subjects, intervention group (probiotic administration) and control group (oral normal saline administration). The inclusion criteria were preterm infants born at < 36 weeks, birth weight 1000 to 2500 gr and postnatal birth weight less than or equal to two weeks with intestinal feeding. Exclusion criteria were infants with major congenital malformations (including congenital heart disease, gastrointestinal obstruction, gastroshisis, etc.), congenital metabolic errors, hypoxic ischemic encephalopathy (HIE) of grade two or more according to Sarnat scoring system (17), death in the first 72 hours of life, newborns of addicted mothers, postpartum age more than two weeks and parental unacceptance. The sampling method was convenience sampling (random appointment). Randomized randomization method and four blocks were used.
In this study, after selection of newborns with entry criteria, all patients received standard treatment, were breastfed and then randomly assigned to one of the intervention or control group. Dosage, frequency and duration of Probiotics in this study was based on current practices of probiotic use in the NICU (18). In the intervention group, after reaching the infant’s volume of 1 cc/kg/day, oral probiotics with a dose of 1 drop/ kg diluted with up to 0.5 cc saline solution and in the control group 0.5 cc, a normal saline solution were added to mother’s milk in every 12 hours and continued to reach 120 cc per kilogram for body weight (full feeding). Fresh suspensions of supplements were individually prepared under strict asepsis by study nurses who were not directly involved in routine patient care for each study infant. The placebo looked identical to the probiotics. The increasing amount of daily milk was similar in both groups. The medication and placebo solution were prepared in same pre-prepared syringes by nurses and infants were evaluated by the assistant who was not aware of the type of medication given. A probiotic drop containing Lactobacillus rhamnosus, Bifidobacterium infantis, and Lactobacillus reuteri (Pedilact, Zist-Takhmir, Iran), which contained Lactobacillus rhamnosus ATCC 15820 (1 × 1010 colony-forming unit [CFU]/mL), Lactobacillus reuteri ATCC 55730 (2 × 109 CFU/mL), and Bifidobacterium longum subsp. infantis ATCC 15697 (1.5 × 109 CFU/mL) (Pedilact, Zist-Takhmir, Iran) was used in this study.
Finally, the data were recorded by the data collection form. The instrument used in this study included 14 questions about age, gestational age, mode of delivery, gender, birth weight, discharge time, kind of feeding, cause of hospitalization, incidence of complications and time to full intestinal feeding in the two groups. The data were entered into SPSS 21 software. Descriptive statistics (mean, standard deviation, frequency) and analytical statistics (independent t-test, chi-square) were analyzed, and P < 0.05 was considered significant.
4. Results
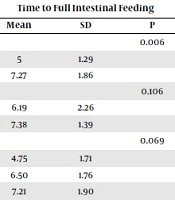
The demographic data of the infants are presented in Table 1. The mean gestational age of infants was 32.48 ± 2.87. The average weight of infants was 1740.43 ± 514 gr and mean day of feeding was 3.13 ± 1.94 days. 77.7% of births were cesarean birth (CD) and 22.4% were normal vaginal delivery (NVD). There was no significant difference in demographic characteristics of newborns between the intervention and placebo groups (P > 0.05). Comparision of time to full intestinal feeding in the two groups showed that, the mean and standard deviation in the intervention group were 5.7 ± 1.96, while in the placebo group was 6.72 ± 1.98, this difference was statistically significant (P = 0.002) (Table 2). Resuls of Table 3 showed that relation between time to full intestinal feeding and study variables (gender, type of nutrition, gestational age and birth weight) were not statistically significant (P < 0.05). However, in the placebo group, time to full feeding in cesarean delivery had a higher mean and standard deviation than vaginal delivery, this difference was statistically significant (P = 0.006). Comparison between intervention and placebo group showed that, time to full intestinal feeding was significantly different only in mode of delivery (P = 0.029).
| Study Groups | Intervention Group, No. (%) | Placebo Group, No. (%) | Total, No. (%) | P Value |
|---|---|---|---|---|
| Gender | 0.59 | |||
| Male | 18 (62.1) | 16 (55.2) | 34 (58.6) | |
| Female | 11 (37.9) | 13 (44.8) | 24 (41.4) | |
| Gestational age (w) mean ± SD | 32.93 (2.78) | 31.86 (2.90) | 32.40 (2.87) | 0.15 |
| Birth weight (gr) mean ± SD | 1857.5 (498.11) | 1623.28 (511.56) | 1740.43 (514.19) | 0.083a |
| Mode of delivery | 0.753 | |||
| NVD | 6 (20.7) | 7 (24.1) | 13 (22.4) | |
| CS | 23 (79.3) | 22 (75.9) | 45 (77.6) | |
| Cause of hospitalization | 0.585 | |||
| Prematurity | 9 (31) | 11 (37.9) | 20 (34.5) | |
| Respiratory distress | 20 (69) | 18 (62.1) | 38 (65.5) | |
| Type of nutrition | 0.490 | |||
| Breast milk | 6 (20.7) | 4 (13.8) | 10 (17.2) | |
| Formula | 3 (10.3) | 6 (20.07) | 9 (15.5) | |
| Combination | 20 (69) | 19 (65.5) | 39 (67.2) |
aChi-square
| Study Groups | P Value | |||
|---|---|---|---|---|
| Intervention | Placebo | Total | ||
| Time to full intestinal feeding (mean ± SD) | 5.07 ± 1.96 | 6.72 ± 1.98 | 5.90 ± 2.12 | 0.002 |
| Study Variables | Study Groups | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention Group | Placebo Group | Total | |||||||
| Time to Full Intestinal Feeding | Time to Full Intestinal Feeding | Time to Full Intestinal Feeding | |||||||
| Mean | SD | P | Mean | SD | P | Mean | SD | P | |
| Mode of delivery | 0.453 | 0.006 | 0.029 | ||||||
| NVD | 4.50 | 2.74 | 5 | 1.29 | 4.77 | 2.01 | |||
| CS | 5.22 | 1.76 | 7.27 | 1.86 | 6.22 | 2.07 | |||
| Gender | 0.6 | 0.106 | 0.352 | ||||||
| Male | 5.22 | 2.16 | 6.19 | 2.26 | 5.68 | 2.23 | |||
| Female | 4.82 | 1.66 | 7.38 | 1.39 | 6.21 | 1.98 | |||
| Type of nutrition | 0.476 | 0.069 | 0.110 | ||||||
| Breast milk | 4.67 | 1.86 | 4.75 | 1.71 | 4.70 | 1.70 | |||
| Formula | 4 | 1 | 6.50 | 1.76 | 5.67 | 1.94 | |||
| Combination | 5.35 | 2.08 | 7.21 | 1.90 | 6.26 | 2.19 | |||
| Gestational age (w) | 0.053 | 0.865 | 0.059 | ||||||
| > 32 | 5.85 | 2.23 | 6.78 | 2.16 | 6.39 | 2.20 | |||
| < 33 | 4.44 | 1.50 | 6.64 | 1.75 | 5.33 | 1.92 | |||
| Gestational weight (gr) | 0.991 | 0.370 | 0.559 | ||||||
| 1000 - 1500 | 5 | 1.56 | 7 | 2.07 | 6.20 | 2.10 | |||
| 1501 - 2000 | 5.10 | 2.23 | 7.17 | 1.17 | 5.88 | 2.13 | |||
| 2001 - 2500 | 5.11 | 2.26 | 5.88 | 2.23 | 5.47 | 2.21 | |||
5. Discussion
In this study, we aimed to detect the effect of probiotics on time to full intestinal feeding of preterm infants. The results of this study showed that there is a positive relationship between the feeding with probiotics and time to full feeding that means infants receiving probiotics reached full feeds earlier, which was similar to the result of other studies (19, 20). Results of another trial on neonates with a gestational age of 28 - 34 weeks and birth weight of 1,000 - 1,800 gr showed that no significant intergroup differences were found in time to reach full feeding (21). The beneficial role of decreasing feeding tolerance by shortening the time to achieve full intestinal feeding is suggested in a systematic review study (22), so the optimization of intestinal feeding is a priority in preterm infants. Early full feeding plays an important role in development of preterm infants, and feeding intolerance often leads to many serious problems that threat the survival of them. Preterm infants with feeding intolerance have an inability to digest enteral feeding (23) and feeding tolerance is frequently reduced in preterm infants. Our results showed that feeding intolerance did not occur in probiotic group, which was similar to the result of a recently published study (10). Probiotic improves gastrointestinal motility which probably accounted for improvement in feed tolerance in probiotic group.
However, in our study we compared birth weight (P = 0.083) and weight at discharge (P = 0.196), that shows there were no significant differences in weight variation (P = 0.437) in each group. One other study also demonstrated similar results, that weight gain was not affected by probiotic supplementation (24). However results of a similar studies in preterm infants born at less than 34 weeks showed that the mean weight gain of newborns in probiotic group was significantly higher than control group (14, 25). This difference can be due to the type and amount of probiotics used in the two studies. Also weight gain in preterm infants is affected by coexistent morbidities such as provision for total parental supplementation and breast milk feeding which possibly affected the weight in these studies. A recent study in preterm infants with birth weight less than 1000 g showed that weight gain was similar in the study groups, but infants had a better cranial growth rate during the first month of life (26). However, we did not collect data relevant to these aspects.
According to the findings, there was no significant correlation between study variables and time to full intestinal feeding except mode of delivery (in vaginal births, the average time to full intestinal feeding is lower than in cesarean delivery). The community composition of neonate gut bacteria can be altered by the mode of delivery (27, 28). It has been reported that infants who are born by vaginal delivery having a similar microbiota to that of their own mothers, contain Lactobacillus in their GI tract (29).
Concerns regarding safety issues and complications associated with the use of probiotics in preterm infants have been debated. However, in our study, no adverse effects were observed in the two groups, this result is in line with previous studies in preterm infants (30, 31). Although sepsis due to translocation of the probiotics through the intestinal wall is extremely rare, some studies have reported sepsis occurring due to organisms present in probiotics (32, 33).
Establishment of full intestinal feeding is a major challenge in the care of preterm low birth weight infants. Feeding with probiotics is recognized as an effective way to prevent adverse health outcomes in preterm infants. Although probiotics efficacy in reducing infants mortality and morbidity was found, more studies are needed to address safety issues and also to answer to the questions as to which probiotic to use, at what dosage, and how long to supplement preterm infants.
This study has some limitations. We focused on clinical manifestations of preterm infants which described that feeding intolerance could decrease with prescribing probiotics, while the characteristics and variation in gut microbial composition of preterm infants is not detected. However, we did not collect data relevant to these aspects. Also including time restriction, we chose few cases, and complicated cases were not enrolled in this study.