1. Background
Neonatal hyperbilirubinemia is a very abundant, multifactorial, and quite challenging disorder resulting from elevated total serum bilirubin (TSB) level which is experienced within the neonatal period, particularly in the first week of life (1). This extremely prevalent medical problem affects approximately 8% - 11% of symptomatic infants who have the yellow coloration of the skin and sclera of the eyes caused by bilirubin (1). Currently, two clinically safe and effective treatment approaches are used in the most benign neonatal jaundice cases for reduction of the acute high bilirubin blood level, which are phototherapy and the acceptable rapid decrement method of exchange transfusion (2-4). According to the neonatal jaundice care guideline, both of these methods must be timely applied at total bilirubin serum level or when exceeding it, depending on the threshold of phototherapy and ECT treatments to avoid causal over-treatment complications (5, 6). Furthermore, all healthy term newborns with severe jaundice should be screened for simultaneous presence of the recurrence and exacerbation of persistent hyperbilirubinemia and potential high-risk presence of significant short-term and long-term side effects expressed with exchange transfusion therapy (2, 7). ECT therapy is the most common medical procedure for conditions that require immediate clinical intervention such as preterm infants, breast milk and feeding jaundice, ABO isoimmunization, and glucose-6-phosphate dehydrogenase (G6PD) inconsistencies (7). According to the pooled data from hospital-based case control studies, almost in two-thirds of these cases, ECT was not in a good condition and even newborn death could occur within seven days after the transfusion in up to 10 percent of the severe cases (8). In other words, because of the increasing adverse effects of significant complications and severe risk factors accompanied by blood exchange, this procedure is considered as the most frequent documented cause of infants’ fatality, with the reported incidence rate of about 10 to 74 percent (9). Some of the most often clinical events submitted to ECT were thrombocytopenia (44%), hypocalcemia (29%), and metabolic acidosis (24%), of which 69%, 74%, and 44%, respectively needed rescue treatment (10). Besides, serum sodium metabolic abnormalities together with calcium homeostasis disturbances (hypocalcemia) and thrombocytopenia are frequently observed in routine clinical practices (11). In other words, ECT is the primary identified contributing factor for the increased risk of encountering harmful hyperbilirubinemia recurrence in 18% of term newborns and it increases mortality in preterm infants and infants with other comorbidities (11).
Many previous studies have reported that the most important reason of the apparent sodium ion concentration change near the upper limit of standard value in most cases, during and after exchange blood transfusion, is poor nutrition in the first several days of life that leads to physiological loss of extracellular fluid’s volume, body water imbalance, and dehydration (5, 12-14). Additionally, serum sodium ion concentration disturbances following administration of an extensive quantity of stored blood products, which are typically sodium bicarbonate-rich solutions, could be deduced as poor prognosis of renal failure (15, 16). Shared clinical symptoms of many primary diseases in infancy such as meningitis, cardiac arrhythmias, sepsis, and congenital metabolic disorders, as well as hypoglycemia and hypocalcemia, can be mimicked by medical signs associated with sodium alterations (17). Therefore, preventing more severe abnormalities due to unnatural serum sodium ratio changes, especially neurological dysfunction and precipitation of severe heart failure and arrhythmia, could equally be supported by timely professional medical care interventions through ensuring well-organized treatment of transfusion-dependent patients (18, 19). There are a few quantitative and qualitative surveys conducted for determining and modifying the increased risk of detrimental clinical outcomes correlated with possible fatal sodium abrupt changes during and after the exchange transfusion in newborns.
2. Objectives
Therefore, this study aimed to investigate the effectiveness of monitoring sodium disorders and the required interventions indicating the overall state of health of hospitalized jaundiced infants.
3. Methods
3.1. Experimental Pattern and Subjecting Technique
This prospective analytic study was performed on 49 neonates diagnosed with features of neonatal jaundice who were admitted to the neonatal ward of Abouzar Hospital in Ahvaz, Iran in order to undergo screened exchange blood transfusion according to the protocol authorized by the transfusion medicine unit of the hospital in a period from November 2018 to May 2019.
More specialized primary practices regarding pediatric healthcare were performed by physician assistants, including the critical need for blood exchange. Serum sodium ion concentration test of each of the desired samples was performed in a laboratory at three recommended points in time: Before, 1 - 3 hours after, and then 24 hours following blood exchange using the most sensitive and specific ELISA kits. In the next step, the collected data related to measured parameters was used to compare the considerable differences in these three times alongside other etiological risk factors. In this study, necessary hospital interventions and functional follow-up visits to patients who suffered from a severe disorder were done based on their presentation.
The ethics committee of Jundishapur University of Medical Sciences approved this clinical research (code: IR.AJUMS.REC.1398.541), and written informed consent was taken from all the parents or guardians of the subjects.
3.2. Statistical Analysis
The Kolmogorov-Smirnov test was used to examine the normal distribution of information. The statistics related to collected data with quantitative values were reported as mean, median, and standard deviation. In contrast, descriptive statistics involving frequency, the relative frequency, and percentage distribution were computed as appropriate summary measures for categorical or qualitative data. In the parametric multivariate analysis using the newest version of SPSS 24 software program, a probability-calculating technique for determining statistical strength of the correlation between different numerical quantitative variables was assessed using a repeated-measures ANOVA test. The P < 0.05 criterion was set as the threshold of significance.
4. Results
4.1. Fundamental Individual Dataset and Specific Clinical Evidence of the Research Output Affiliated to the Experimental Trial
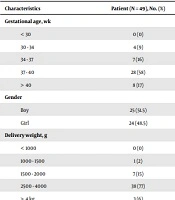
Sub-groups of the general clinical features of this study’s patients are demonstrated in Table 1. As shown there, in a total of 49 infants, 24 (48.5%) were girls and 25 (51.5%) were boys. The gestational ages for the statistical recording of neonatal jaundice varied from eight (17%) for more than 40 weeks, 28 (58%) for 37 to 40 weeks, 7 (16%) for 34 to 37 weeks, to four (9%) for 30 to 34 weeks. Birth weights of newborns were classified as 38 (77%) for babies that weighed between 2,500 to 4,000 g, 7 (15%) for those who weighed 1,500 to 2,000 g, and 1 (2%) for those weighing between 1,000 and 1,500 g, while, three (6%) was attributed to the newborns that weighed more than 4 kg (Table 1). The mean length of hospital stay of newborns of all the hospital deliveries was 4.55 ± 2.399 days. All neonates were clinically followed up for a maximum period of four days and exchange transfusion treatment and related therapeutic modalities were completed in four (8%) hospitalized patients within a maximum length of 10 days.
| Characteristics | Patient (N = 49), No. (%) |
|---|---|
| Gestational age, wk | |
| < 30 | 0 (0) |
| 30 - 34 | 4 (9) |
| 34 - 37 | 7 (16) |
| 37 - 40 | 28 (58) |
| > 40 | 8 (17) |
| Gender | |
| Boy | 25 (51.5) |
| Girl | 24 (48.5) |
| Delivery weight, g | |
| < 1000 | 0 (0) |
| 1000 - 1500 | 1 (2) |
| 1500 - 2000 | 7 (15) |
| 2500 - 4000 | 38 (77) |
| > 4 kg | 3 (6) |
| Causes of jaundice | |
| Idiopathic | 22 (45) |
| ABO isoimmunization | 11 (23) |
| Rh isoimmunization | 10 (20) |
| Breast milk | 1 (2) |
| Breast feeding | 4 (8) |
| G6PD inconsistencies | 1 (2) |
Only a 3-day-old 3,100 g male infant born at 37 weeks’ gestation had G6PD deficiency - leading cause of jaundice without manifestation of severe bacterial sepsis or other pathological detectable drivers of neonatal jaundice. Jaundice was observed a few days after delivery in four male breastfed term newborns with birth weights between 2,500 and 4,000 g, and gestational ages of 39 to 40 weeks. No dissimilarities were identified in the neurological investigation of these newborns. A 3,100 g 38-week gestational age girl was identified with breast milk jaundice in the first week after birth (the seventh day of life), while her medical examinations were normal. The observational trial of this study suggested that the fourth case that had ABO incompatibility and received double exchange transfusion due to acute hyperbilirubinemia, did not have comparatively lower bilirubin serum level, and one exchanged case notably had the highest bilirubin level only one day after, which was more than 25 mg/dL. Further exchange transfusion courses were performed mainly in 10 infants aged four days and at raised total serum bilirubin level of 28 mg/dL.
4.2. The Account of Various Factors, Particularly Numerous Causes Leading to Jaundice, Different Birth Weights, and Actual Gestational Age Associated With Post-Exchange Serum Sodium Levels Disturbances
All the newborn infants with jaundice in this study that suffered from a broad range of clinical issues and had different birth weights and distinct gestational ages demonstrated significant mean sodium serum level declines at 1 to 3 hours and 24 hours after exchange transfusion in comparison to the time before the exchange.
Despite a diminished sodium concentration, the mean sodium levels stayed within the laboratory reference range of the serum (137 - 142 mEq/L) at the three points in time mentioned above. In addition, variation in the amount of serum sodium was associated with an unknown underlying cause that led to neonatal jaundice (P = 0.04, Table 2) and this difference did not reach statistical significance in various trends in birth weight, gestational ages, and other causes accounting for high bilirubin (P > 0.05). The most common changes of the mean value in blood sodium level were noticeably found in infants aged six days. The highest bilirubinemia risk, which was roughly 26 mg/dL serum bilirubin, was observed in the icteric newborns that had low maximum serum sodium (138 mEq/L).
| Causes of jaundice | Before | 1 - 3 h | 24 h | |||
|---|---|---|---|---|---|---|
| Mean ± SD | P-Value | Mean ± SD | P-Value | Mean ± SD | P-Value | |
| Idiopathic | 142 ± 3.72 | 0.251 | 137 ± 1.32 | 0.047 | 135 ± 0.4 | 0.04 |
| ABO isoimmunization | 146 ± 3.89 | 0.103 | 137 ± 1.39 | 0.210 | 136 ± 0.2 | 0.12 |
| Rh isoimmunization | 140 ± 3.54 | 0.251 | 137 ± 1.77 | 0.125 | 136 ± 0.77 | 0.302 |
| Breast milk | 138 ± 0.02 | 0.147 | 136 ± 1.14 | 1.02 | 135 ± 1.06 | 0.08 |
| Breast feeding | 137 ± 0.66 | 0.201 | 136 ± 1.16 | 0.951 | 135 ± 0.73 | 0.092 |
| G6PD inconsistencies | 137 ± 0.91 | 0.061 | 136 ± 0.4 | 0.852 | 136 ± 0.22 | 0.056 |
aP-value < 0.05 was considered statistically significant. Repeated-measures ANOVA test was used to compare the relationship between different means for normally distributed variables.
5. Discussion
A less aggressive exchange transfusion is capable of getting rid of extreme neonatal hyperbilirubinemia. This potentially emergency practice is regularly utilized in term infants for suppressing the occurrence of acute bilirubin encephalopathy (ABE), kernicterus features, and death (20). Reversible electrolyte imbalances, especially natural serum sodium and potassium disturbances, are the two most increasingly frequent abnormalities that are referred for administration of ECT therapy (14). To the best of the authors’ knowledge, there are a few conducted quantitative and qualitative surveys for determining and improving the increased risk of detrimental clinical outcomes correlated with possible fatal abrupt changes in sodium during and after the exchange transfusion in newborns. Therefore, this study was aimed to examine the effectiveness of monitoring sodium disorders and the required interventions regarding the overall state of health of hospitalized jaundiced neonates.
At first, primary stratified findings based on the results of this original research on patients who had ECT, demonstrated a declining trend in serum sodium concentration in neonates, but these changes were outside an abnormal range of expected values. The same observations were reported in the prospective study of Esmaeilivand et al. (21), which delineated decreased sodium levels after ECT in 8% of neonates with serious jaundice. Despite their interventions, hyponatremia was detected in some of the jaundiced neonates and serum sodium levels did not elevate (22). Similarly, the most recent literature has proposed that this method does not carry much risk for serum sodium imbalance in the situation of jaundice in newborn patients. For example, Calladine et al. (23) and Ballot and Rugamba (5) found that the mean sodium levels remained within the normal range directly after the ECT process in neonatal hyperbilirubinemia, which is consistent with the results of the present study. In comparison with these pilot feasibility studies and the current study, a large number of clinical transfusion trials have provided evidence that a considerable surge in serum sodium average in neonates and higher incidence of hypernatremia have happened during and after blood exchange, in contrast to the time before the exchange transfusion. In addition, Jurfest-Ceccon et al. (24) showed that an increasing frequency in serum sodium levels of about 2.8% to 3.2% was observed during and after ECT in icteric neonates. Furthermore, alteration in the level of serum sodium was not observed in the study of Wani et al. (8), in which they concluded that a heedful exchange transfusion strategy did not support this negative impact. Such dramatically different data across clinical practice fields related to neonatal jaundice and various researches that were mentioned above can be attributed to the differences in the clinical status of term neonates and survey periods. Given these facts, it is concluded that neonatal jaundice is significantly associated with changes in serum sodium level after receiving ECT, based largely on a baby’s age and mean total serum bilirubin levels.
5.1. Conclusions
In summary, notwithstanding the highly therapeutic effectiveness of ECT modality as a suitable alternative treatment option for neonatal jaundice (NJ), this approach has its dire consequences. Jaundiced neonates were most susceptible to electrolyte imbalance panel, which is the tendency for rapid decline in serum sodium levels. Certainly, future studies with larger populations and longer follow-up periods are needed that can directly lead to further examination of significant negative electrolyte changes coincided with ECT therapy and long-term follow-up treatment of infant patients showing the characteristics of jaundice. Therefore, the task of differential diagnosis of direct ongoing health side-effects produced by ECT medication in icteric neonates could be effective in early diagnosis of relative clinical manifestations and indications and inhibiting several principal causes of elevated morbidity or mortality rates that occur due to sodium disturbances and other serious complications.