Abstract
Context:
Congenital heart disease (CHD) is a leading cause of mortality by birth defects with significant social and economic burden. Pulse oximetry as a safe and non-invasive screening method, and with its potential for early detection of CHD has improved neonatal health outcomes.Objectives:
The aim of this study was to systematically review economic evaluation studies that compared pulse oximetry with current programs to diagnose early detection of CHD in full-term newborns.Data Sources:
A systematic review was conducted according to preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines, and related articles published from 1995 up to March 2020 were searched in different databases (MEDLINE, EMBASE, PubMed, Science Direct, Google Scholar, Scopus, NHS EED, Science Citation Index, MagIran, Cochrane Library, EconLit and SID). The articles were selected based on inclusion and exclusion criteria. Consolidated health economic evaluation reporting standards (CHEERS) statement checklist was used to qualitatively evaluate the papers. Overall, 7 articles were included in the study.Results:
Timely diagnosis was considered as main effectiveness health outcome in most studies. The highest and lowest values of incremental cost-effectiveness ratio (in two-phase studies) were €139,000 and $100 per infant in the Netherlands and Colombia respectively; and (in one-phase studies) were £24,000 and £1,489 per infant both belonging to the UK. Implementing pulse oximetry method concurrent with the clinical examination is more cost-effective. The reviewed studies had been conducted in high-income and upper middle-income countries; therefore, when the results are generalizing by policy makers in different health systems, a substantial precaution approach is needed.Keywords
Cost-Effectiveness Analysis Economic Evaluation Congenital Heart Defect Neonatal Screening Pulse Oximetry Systematic Review
1. Context
CHD is the leading cause among mortality causes by birth defects in children under 5-years-old; more than 9 million died in 2016 due to CHD; and constrained a major impact on both the lives of children and their families (1, 2). Close to 25% of these children generally need surgical intervention or other procedures in the first year of life and without intervention it can lead to significant morbidity and mortality (3). The current worldwide incidence of CHD ranges from 8 to 12 per 1000 live births (4). Pediatric critical CHD (CCHD) associated with hospitalization had the highest portion ($5.6) of total hospital costs for cardiovascular defects ($6.1 billion) in 2009 (5, 6).
Delayed diagnosis of CHD could be associated with sudden cardiac failure, cardiovascular collapse, organ damage, and death (7). Through routine assessments, up to 39% of infants with CCHD will leave the hospital undiagnosed and about 43% of them will return to the hospital in hemodynamic instability or shock status, some may die at home without any diagnosis or endure major morbidity (8-10).
Critical CHD refers to lesions requiring surgery or catheter-based intervention in the first year of life. This category includes ductal-dependent and cyanotic lesions as well as less severe forms of CHD that are not dependent on the patent ductus arteriosus (11). In recent years, pulse oximetry identified as a diagnostic method that can detect cyanogen anomalies in patients with mild hypoxemia (O2 saturation 85% - 95%), which cannot be detected by clinical examination. Additional diagnostic tests (echocardiography) confirm or exclude the existence of CHD. Of crucial importance is the optimal time of diagnosis; this especially applies to cyanogen anomalies that require immediate treatment (12). Screening is performed by qualified and trained personnel and oxygen saturation (SpO2) is measured in either the right hand (pre-ductal) or foot (post-ductal). Screening at both locations can occur simultaneously or in direct sequence. Post-ductal measurement of SpO2 is important because defects with right-to-left shunting of desaturated blood through the ductus arteriosus will not be detected with only pre-ductal measurement. A cutoff SpO2 value of < 95 percent is used as it provides a sensitivity of around 75 percent and specificity > 99 percent (11-14).
Pulse oximetry, during last decades, has been considered as a simple, painless, fast, inexpensive and non-invasive method that represents the percentage of oxygenated hemoglobin in the blood and shows some hypoxia scales in severe cyanotic heart disease which are not clinically apparent (2, 15-18). Making decision on health economic issues is a major concern and ultimately leads to cost management, efficiency enhancement, and optimal allocation of limited funds. Hence conducting an economic evaluation on diagnostic methods for CHD has a critical role during infancy.
2. Methods
Cost-effectiveness studies that assessed the direct medical costs on pulse oximetry screening for congenital heart disease in full-term newborns were identified through a pragmatic literature review conducted in different databases includeing MEDLINE, EMBASE, PubMed, Science Direct, Google Scholar, Scopus, National Health Service Economic Evaluation Database (NHS EED), European Network of Health Economics Evaluation Databases (EURONHEED), Science Citation Index (Web of Knowledge), EconLit, The Cochrane Library (Cochrane Database of Systematic Reviews(CDSR)), Cochrane Methodology Register, Database of Abstracts of Reviews of Effects (DARE) and Health Technology Assessment (HTA) Database, MagIran and SID from 1995 up to March 2020 in English and Persian language by using a combination of key and MeSH Terms: Cost-Effectiveness Analysis (CEA), Cost-Utility Analysis (CUA), Cost-Benefit Analysis (CBA), Economic Evaluation, Congenital Heart Disease, Neonatal Screening and Pulse Oximetry.
2.1. Inclusion Criteria
Studies were included
• If they focused on the use of pulse oximetry in mature infants.
• Original studies that performed a full economic evaluation (including CEA, CUA, and CBA).
• Implemented models like the Markov model or analytic decision tree.
• Reported QALY, LYG, DALY, the number of timely detected or additional timely detected cases (TD/ATD) and ICER.
• Papers published during the years from 1995 up to March 2020, with available full texts.
• Papers published in English or Persian language.
2.2. Exclusion Criteria
Studies were excluded if they
• Were designed as partial economic evaluation (such as those evaluating effectiveness, cost saving analysis (CSA) and cost analysis (CA)), or assessed the quality of life.
• Not considered as actual cost-effectiveness analysis (methods or protocol papers).
• Assessed the prenatal examinations as a screening method to detect CHD.
• Assessed the pre-term infants to detect CHD.
• Conference paper abstracts where full analysis was not available, case reports, case series, review, abstract, and editors.
• Lacked sufficient quality of methodology or having inadequate quality based on the CHEERS checklist.
2.3. Quality Assessment of the Studies
The quality of the methodology of the studies was evaluated using the CHEERS checklist to assess the methodological quality of the selected studies (19). Any disagreement between the two authors was resolved through the involvement of a third author, who acted as the final referee.
Items of the checklist that were completely met in the studies received a score of 1 and marked with the symbol of “Y”; items that were partially met in the studies received a score of 0.5 and marked with the symbol of “P”, and items that were not fulfilled at all received a score of zero and marked with the symbol of “N”. Studies with a score above 85% have been considered as excellent quality, studies with a score of < 70% - 85% as very good quality, papers with a score of < 55% - 70% as good quality, and articles with a score of less than 55% were classified as poor quality. Based on the results, 3 articles received a score above 85% and had “excellent” quality and 4 articles with a score of 70% - 85% rated as “very good” ones (Table 1).
CHEERS Checklist- The Quality of Methodology of the Studies
| CHEERS Items | Item No | References | ||||||
|---|---|---|---|---|---|---|---|---|
| Griebsch et al. (2007) (20) | Roberts et al. (2012) (21) | Peterson et al. (2013) (22) | Tobe et al. (2017) (23) | Narayen et al. (2018) (24) | Mukerji et al. (2019) (25) | Trujillo et al. (2019) (26) | ||
| Title | 1 | Y | Y | P | P | Y | Y | Y |
| Abstract | 2 | P | Y | P | Y | P | Y | Y |
| Background and objective | 3 | Y | Y | Y | Y | Y | Y | Y |
| Target population and subgroup | 4 | Y | Y | Y | Y | Y | Y | Y |
| Setting and location | 5 | P | Y | Y | Y | Y | Y | Y |
| Study perspective | 6 | Y | Y | P | Y | Y | Y | Y |
| comparators | 7 | Y | Y | Y | Y | Y | P | Y |
| Time horizon | 8 | P | P | Y | Y | P | Y | Y |
| Discount rate | 9 | Y | NA | NA | P | Y | P | NA |
| Choice of health outcomes | 10 | Y | P | Y | Y | Y | Y | P |
| Measurement of effectiveness (single study-based estimates) | 11a | Y | Y | Y | Y | Y | NA | NA |
| Measurement of effectiveness (synthesis-based estimates) | 11b | NA | NA | NA | NA | NA | P | Y |
| Measurement and valuation of preference-based outcomes | 12 | NA | Y | NA | NA | Y | Y | NA |
| Estimate resources and cost (single study-based economic evaluation) | 13a | NA | NA | N | NA | NA | NA | NA |
| Estimate resources and cost (model-based economic evaluation) | 13b | Y | Y | Y | Y | Y | P | N |
| Currency, price date, and conversion | 14 | P | Y | Y | Y | Y | Y | P |
| Choice of model | 15 | Y | Y | Y | Y | Y | Y | Y |
| Assumptions | 16 | Y | Y | Y | N | Y | Y | Y |
| Analytic method | 17 | P | P | P | P | P | P | P |
| Study parameters | 18 | Y | P | N | P | P | P | P |
| Incremental costs and outcomes | 19 | Y | Y | Y | Y | Y | P | Y |
| Characterizing uncertainty (single study-based economic evaluation) | 20a | NA | Y | NA | NA | NA | NA | NA |
| Characterizing uncertainty (model-based economic evaluation) | 20b | Y | NA | Y | Y | Y | Y | Y |
| Characterizing heterogeneity | 21 | Y | NA | NA | NA | NA | NA | NA |
| Study funding. limitation, generalizability, and current knowledge | 22 | Y | Y | Y | Y | Y | Y | P |
| Source of funding | 23 | Y | Y | Y | Y | N | N | Y |
| Conflict of interest | 24 | Y | Y | Y | Y | Y | Y | Y |
| Total percentage | 0.88 | 0.93 | 0.8 | 0.86 | 0.84 | 0.8 | 0.83 | |
3. Data Extraction
After selecting the studies, two authors independently extracted relevant information. The key features included the following: the surname of the first author, year of publication, country of study, study population, mean age of patients, alternative options for comparison, outcome measure, time horizon, study model, study perspective, cost, type of sensitivity analysis, discount rate for cost, effectiveness, and ICER (Tables 2 and 3).
Description of Cost-Effectiveness Study Characteristics
| Study, Publication Year | Country | Number of Patients | Mean Age | Study Perspective | Time Horizon | Health Outcomes | Sensitivity Analysis | Discount Rate | Objective |
|---|---|---|---|---|---|---|---|---|---|
| Griebsch et al., 2007 (20) | UK | 100,000 | First 24 hour | Health care payer | 1 years | TD | Probabilistic sensitivity analysis | No | To investigate the effectiveness, costs, and cost-effectiveness of adding PO or screening ECHO to the current strategy of CE to inform future screening policy. |
| Roberts et al., 2012 (21) | UK | 20,055 | First 48 hour | Health care payer | 1 years | TD | Probabilistic sensitivity analysis | No | To undertake a cost-effectiveness analysis that compares PO as an adjunct to CE with CE alone in newborn screening for CHDs. |
| Peterson et al., 2013 (22) | USA | 3.952.138 | First 24 hour | Health care payer | < 1 year | Lys | One-way sensitivity analysis | No | To estimate the cost effectiveness of routine screening among US newborns unsuspected of having CCHD¤. |
| Tobe et al., 2017 (23) | China | 16,000,000 | First 48 hour | Societal | Lifetime | DALY | One-way & multivariate probabilistic sensitivity analyses | 3% | To evaluate the cost-effectiveness of neonatal CCHD screening for neonates in China. |
| Narayen et al., 2018 (24) | Netherlands | 23,959 | First 48 hour | Health care payer | < 1 year | ATD | One-way sensitivity analysis | No | To estimate the additional costs of PO in the Dutch perinatal care system, plus personnel time and equipment. |
| Mukerji et al., 2019 (25) | Canada | 150,000 | First 24 Hour | Health care payer | Lifetime | - ATD -QALM | Probabilistic sensitivity analysis | 1.50% | To estimate the cost-effectiveness of POS for CCHD in Ontario, Canada. |
| Trujillo et al. (2019) (26) | Colombia | A hypothetic cohort of mature newborns | First 24 Hour | Societal | < 1 year | TD and LYS | One-way probabilistic sensitivity analysis | No | To assess the cost effectiveness PO plus CE in timely detection of CCHDs compared with CE and its budget impact, as a new national policy |
Studies’ Indices of Economic Evaluation
| Study | Price/Year | Study Model | Comparators | Threshold | Health Outcomes | ICER | Cost | Results |
|---|---|---|---|---|---|---|---|---|
| Griebsch et al. (2007) (20) | UK (£)/2000-2001 | Decision-analytic tree | CE; CE + PO; CE + ECHO | £50,000 | Primary outcome: CE: 34.0, PO: 70.6, ECHO: 71.3; Secondary outcome: CE: 222.4, PO: 342.2, ECHO: 427.4 | Primary outcome: PO: £ 4,894; ECHO: £4,496,66; Secondary outcome: PO: £1,489, ECHO: £36,013 | Primary outcome: CE: 296,891, PO: 476,193, ECHO: 3,540,388; Secondary outcome: CE: 297,627, PO: 476,016, ECHO: 3,457,233 | PO vs CE: likely to be cost-effectiveness. ECHO: unlikely to be cost effective, unless the detection of all clinically significant CHD is considered beneficial and a 5 percent false-positive rate acceptable. |
| Roberts et al. (2012) (21) | UK (£)/2009 | Decision-analytic tree | CE; CE + PO | £20,000 | CE: £91.5; PO + CE: £121.4 | £24,000 | CE: £614,000; CE + PO: £ 1358800 | PO as an adjunct to routine practice of CE was likely to be a cost-effective intervention. |
| Peterson et al. (2013) (22) | US($)/2011 | Decision-analytic tree | No screening; ECHO + PO | $50000 - $100000 | ECHO + PO: $30.28 | $20,862 per additional newborn & $40 385 per life-year gained | Cost of screening: $6.28 per infant; Total cost: $24 802 782 | CCHD¤ screening appears cost-effective using conventional thresholds and may be cost-saving under some circumstances. |
| Tobe et al. (2017) (23) | US($)/2015 | Markov model | CE; PO; CE + PO | $34,857 per DALY | CE: 371,67 per DALY; PO: 322,65 per DALY; CE + PO: $426,63 per DALY | CE: $7,528/DALY; PO: Dominated; CE + PO: $56,778 Per DALY | CE: $2,798,053; PO: $3,172,834; CE + PO: $5,918,728 | CE compared to NI is the most cost effectiveness.; PO compared to NI is the most cost effective in short time; CE + PO compared to NI is cost effectiveness |
| Narayen et al. (2018) (24) | EUR(€)/2017 | Markov model | PO + Referralk; CE + Referral | €20,000 | PO + Referral (2 phases): €14,71; -CE + Referral (2 phases): €11,00 | €139,000 | PO + Referral: €1,923,000; CE + Referral: €252,000 | PO screening in the Dutch care setting is cost-effective |
| Mukerji et al. (2019) (25) | CA($)/2017 | Decision-analytic tree & markov model | PO; No PO | $4167 per QALMs OR $50,000 per QALY | PO: CA$554,53 per QALM; No PO: CA$554,50 per QALM | PO: $1,110 per QALM | PO: CA$27.27 (The lifetime cost for PO: CA$284,002); (The lifetime cost for No PO: CA$283,975) | PO is cost-effective |
| Trujillo et al. (2019) (26) | US($)/2017 | Decision-analytic tree | PO; PO + CE | (1 week): $26.292; (1year): $6,408; | CE (1 week): 0,86; PO + CE (1 week): 0,93; CE (1 year): 0,9745; O + CE (1 year): 0,9755 | 1 week: $100; 1 year: $39,050 | CE (1 week): $95; PO + CE (1 week): $102; CE (1 year): $326; PO + CE (1 year): $365 | PO + CE (1 week) is a cost-effective strategy; PO + CE (1 year) considering survival rate, is not cost effective |
4. Results
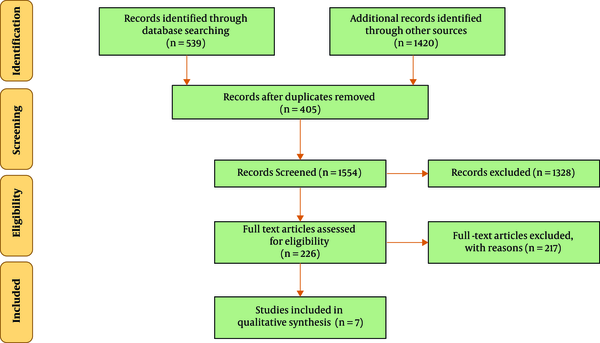
In the initial search of databases, a total of 1959 eligible studies were identified. Of all, 405 studies due to duplication were removed. Keywords, titles and abstracts of the 1554 remaining articles were assessed and 1328 articles that were not related to the topic were excluded. Then a total of 226 related articles were identified for the next phase of the review. After reviewing the full text of the remaining articles, 217 articles were also removed due to failure to meet the exclusion criteria. Of the 9 remaining studies that enrolled into the next phase of study, 2 were excluded due to inadequate report or lack of proper methodology evaluated with the CHEERS checklist. Finally, 7 studies were included. Figure 1 shows an overview of search steps based on PRISMA guidelines.
Process of paper selectin
The general outlines of the articles included in this study are presented in Table 2. The studies come from the United Kingdom (20, 21), the United States (22), China (23), the Netherlands (24), Canada (25) and Colombia (26). Except one study that was published in 2007 (20), the others were published in the recent years (21-26). The target population and their age were almost close. All of the studies focused on the first hours after birth of full-term neonates. The largest population comes from the Chinese study followed by the United States, and all studies together provided approximately 20 million of neonates’ population. All studies have used one-way sensitivity analysis. Four studies used the decision tree (20-22, 26), two studies used the Markov (23, 24) and one study used both the decision tree and Markov model (25). The discount rate has been specified just in two of the studies due to their time horizons (lifetime) (23, 25). There are four types of health outcomes (QALY-LYs-DALY-Timely Diagnoses) in the studies. So in order to come to a comprehensive conclusion, their results are summarized and analyzed together. The Indices of economic evaluation of the studies are presented in Table 3.
Except two studies with societal perspective, most reviewed studies have followed the healthcare sector perspective (20-22, 24, 25), as is conventional for clinical interventions (23, 26). For instance Trujillo et al. investigated the budget impact for utilization of pulse oximetry and showed although health system paid in Colombia is covered by insurance companies, it still had a high economic impact (for CHD) on the Colombian health system and families. They estimated a considerable increase on the budget impact from $2,512,359 to $5,069,018 if the percent of screened newborns with pulse oximetry increases from 10% to 20% (26). In another study with societal perspective, costs of screening methods were estimated based on some items such as salaries of doctors and nurses, average screening time, equipment and its maintenance services like pulse oximetry devices and reusable sensor with wraps and also costs for implementing screening program by Tobe et al. They concluded that in light of China’s low “ceiling” levels on threshold, out of pocket payment and various restrictions on reimbursements, particularly for rural and rural-to-urban migrant children; neonatal screening for detecting CHD imposed additional costs on families and also on the health care sector. They estimated a notable increase in costs from In $2,798,053 to In $3,172,834 when clinical assessment and pulse oximetry were using respectively (23).
Timely diagnosis as health outcome varies from 30 additional timely diagnosis (21) to 70.6 timely diagnosis (20) per 100,000 live births in the UK, twice the proportion detected by clinical examination, and ranged ICERs from £1,489 (20) for using pulse oximetry alone to £24,000 (21) using pulse oximetry in addition to clinical assessment; both of these studies suggested pulse oximetry as a cost-effective screening method. This method was a cost-effective method not only on timely diagnosis but also on saving the number of lives during infancy; for example in Peterson’s study (22), those born through pulse oximetry screening had an ICER of $40,385 per life-year gained with 614 life-years saved annually and $20,862 per case identified with CHD at hospital birth with 1189 additional diagnosed newborns and 20 additional infant deaths averted per year in America. Mukerji’s study (25) showed that using pulse oximetry screening improved QALMs by 0.03, as compared with usual care and led to an overall gain of 3682 QALMs or 307 QALYs per birth and related with an estimated 51 additional cases of CCHD diagnosed in a timely fashion annually and a 92.3% chance of being cost-effective at basic ICER threshold per QALM. And although the lifetime cost to the healthcare payer per individual with pulse oximetry and without pulse oximetry were nearly identical, this service was more cost-effective than usual care services in the Ontario State. In Tobe’s study (23), however pulse oximetry method was much more expensive than no intervention, results showed that using this method not only reduced the burden of the disease by up to 30% with an ICER of Int $5,728 per DALY at the basic threshold but also using pulse oximetry alone compared to no intervention was the dominated strategy per averted DALYs in the short time and using it adjunct to clinical assessment significantly yielded the best health outcomes when threshold raised from $34,857 to $75,000 per DALY.
In two different studies with adoption of a two-step strategy, an improvement in timely additional CHD cases has been approved: Narayen’s study (24) in the Netherlands with two time points (1st day of birth and 3rd day of life) conducted for both of hospital and home births to compare a situation with and without pulse oximetry and resulted in 12 additional newborns detected with pulse oximetry. In Londono Trujillo’s study (26) in Colombia also with two time points (1st day of birth and at the end of first year of life) was conducted to compare pulse oximetry as a complement for the clinical examination versus the clinical examination on its own to detect CHD in newborns and showed that pulse oximetry is a cost-effective strategy in the early diagnosis of CCHDs, while implementing this screening considering survival rate was not cost effective in the threshold defined (US$ 26.292). The reported results in the mentioned studies showed that implementing of pulse oximetry for additional timely detecting of neonatal CHD was a cost-effective method and the highest and lowest ICERs in first phase of two phases annually were €139,000 (24) and $100 (26) per infant respectively, while among the rest five reviewed studies that have been done in one phase or one point of time with similar health outcome, the highest and lowest ICERs belonged to two different studies in the UK with £24,000 (21) - £1,489 (20).
5. Discussion
This study is the first systematical review in the last two decades that has comprehensively analyzed the economic aspects and effectiveness of the pulse oximetry diagnostic method. Most reviewed studies were conducted in high-income countries (USA, Europe, and Canada); however, we also included studies from upper middle-income countries (China and Colombia), which increases the generalizability of review findings. The present study showed that accompanying of clinical examination, the pulse oximetry screening for detecting full-term newborns with CHD is more cost-effective than other current screening services. However regarding to economic level of countries and different health outcomes, summarizing the results creates some challenges due to variation of ICER values reported by the reviewed studies.
This diversity comes from different health outcomes and variable costs. In terms of health outcomes, effectiveness depends on the type of monitoring, skill of performer, time spent on monitoring, type of equipment and measured outcome. For example based on health outcome, in the Tobe’s study (23), DALY has been used as a measure analogous to the QALY, so it should be noted that the “disability” weights used to calculate DALYs refer to any short term or long-term loss of health, therefore it assessed morbidity rather than disability; and researchers did not explain how the DALY estimates were generated.
The wide variability in the costs may relate to the nature of models created, data used, different thresholds, out-of-pocket payments for medical expenses, access to health care and reimbursement. For example lack of access to health care, especially advanced pediatric cardiovascular cares, out-of-pocket payments for these high-level services and various restrictions on reimbursements range from a remarkable difference of 25% to 75% on follow-up in China (23); or in the threshold defined, considering the survival rate in late detection, pulse oximetry strategy created more costs and took it out of being cost effective in Colombia (26). Based on the results, it can be said that the development of infrastructural aspects of screening programs such as using pulse oximetry strategy in the future, its implementation and follow-up within longer time periods, and the increase in the number of patients covered by the service (to increase scale efficiency) in upper-middle income countries will further improve the cost-effectiveness of this safe and non-invasive diagnostic method as compared with no intervention without the need to estimate the indicators and could make this method a dominant option, rather than current strategy (no intervention).
However most of the challenges belonged to middle income countries; there was some unmatched results in some developed ones, for example findings showed that although at basic threshold of €20,000 per gained QALY in the Netherlands, the two points of time screening with pulse oximetry was cost-effective, the ICER of detected babies at 3rd day of birth was remarkably lower than 1st day of life (24). This unexpected difference of ICERs between two time points could be interpreted from two directions. Firstly, it shows a situation in which using early pulse oximetry strategy detects more false positive cases and consequently increases the ICER, secondly it is possible that the number of missing infants with CHD increases at the 3rd day after birth, especially in the Netherlands where home birth delivery contains 18% of all normal deliveries. So it needs to be locally evaluated by much more details to make the interpretation of the results more obvious.
Regarding health outcomes, the results showed that the pulse oximetry diagnostic method improved infants’ QALYs, decreased lost years, reduced unnecessary visits, the short-term morbidity, mortality and length of hospital stay in case of timely diagnosis of CCHD. The findings identified that using the pulse oximetry strategy reduced the prolonged hypoxemia to vital organs and increased surviving and moreover dramatically declined the adverse events, and minimized the cost to the healthcare system by minimizing the effect of ambiguity in steps that need to be taken and would maximize the effect of pulse oximetry method (20-22, 25, 27, 28). In general, studies showed that implementing of pulse oximetry for timely detecting of CHD in neonates on different scenarios (using pulse oximetry alone or adjunct to current screening programs) was cost-effective; these results actually were a rational confirmation on Abouk’s study (29), in which they reported a reduction in early infant deaths from CCHD from 33.4%, with an absolute decline of 3.9%, after states implemented mandatory screening compared with prior periods and states without screening policies in America (5).
Although evidence of these reviewed studies suggested that pulse oximetry was likely to be cost-effective, most of studies investigated this method within a short period and we could not assess the long-term costs and benefits per QALY, which is of high importance for policy makers. Hence it is essential to be more cautious when generalizing the results and utilizing them for making policies; moreover, further studies must be conducted under local conditions especially in cases where the lack of cost effectiveness is attributed to high costs. Taking into account that most of the studies were designed and conducted in high-income and upper middle-income countries; thus, generalizing these results in low- and middle-income countries will be argumentative in different contexts and state of affairs. Therefore, if policy makers intend to implement the pulse oximetry method in their own healthcare systems, they should design and conduct specialized studies according to income level in their local setting with the help of specialists and experts in health economics.
5.1. Limitation
Based on the heterogeneity among these studies in terms of modeling approach, studies time frames, health outcomes considered, assumptions and parameters, limited conclusions can be drawn with regard to the absolute cost-effectiveness. And also due to no calculation of the costs in the reviewed studies, we did not include treatment costs in this analysis, whereas it is estimated that the duration and amounts of hospital admissions would be higher in case of late detection of CCHD. In addition this systematic review was limited by the search approach, only studies written in the English and Persian languages were included.
6. Conclusion
Based on the results of the studies reviewed in this systematic review, clinical care plus pulse oximetry is a cost-effective diagnostic method for detecting congenital heart defects in full-term neonates in high/upper middle-income countries. In general, this type of diagnostic method can help to reduce the third party payers’ expenditures and increase the number of timely diagnosis among neonates and can be an appropriate alternative for the clinical assessments alone and other high expensive methods such as cardiac ultrasound for well-being newborns. So it is worthwhile to design and implement other economic evaluation studies under local conditions in low-income or less developed countries in which naturally associated with some limitations and utilize their results to make the best decisions.
Acknowledgements
References
-
1.
Irina Alina C, Mariana Carmen C. Congenital Heart Disease: Global Burden and Challenges to Eliminate Health Disparities. Annals of Public Health Reports. 2018;2(1). https://doi.org/10.36959/856/487.
-
2.
McClung N, Glidewell J, Farr SL. Financial burdens and mental health needs in families of children with congenital heart disease. Congenit Heart Dis. 2018;13(4):554-62. [PubMed ID: 29624879]. [PubMed Central ID: PMC6105538]. https://doi.org/10.1111/chd.12605.
-
3.
Klausner R, Shapiro ED, Elder RW, Colson E, Loyal J. Evaluation of a Screening Program to Detect Critical Congenital Heart Defects in Newborns. Hosp Pediatr. 2017;7(4):214-8. [PubMed ID: 28250095]. [PubMed Central ID: PMC5924700]. https://doi.org/10.1542/hpeds.2016-0176.
-
4.
Hoffman J. The global burden of congenital heart disease. Cardiovasc J Afr. 2013;24(4):141-5. [PubMed ID: 24217047]. [PubMed Central ID: PMC3721933]. https://doi.org/10.5830/CVJA-2013-028.
-
5.
Arth AC, Tinker SC, Simeone RM, Ailes EC, Cragan JD, Grosse SD. Inpatient Hospitalization Costs Associated with Birth Defects Among Persons of All Ages - United States, 2013. MMWR Morb Mortal Wkly Rep. 2017;66(2):41-6. [PubMed ID: 28103210]. [PubMed Central ID: PMC5657658]. https://doi.org/10.15585/mmwr.mm6602a1.
-
6.
Simeone RM, Oster ME, Cassell CH, Armour BS, Gray DT, Honein MA. Pediatric inpatient hospital resource use for congenital heart defects. Birth Defects Res A Clin Mol Teratol. 2014;100(12):934-43. [PubMed ID: 24975483]. [PubMed Central ID: PMC4422978]. https://doi.org/10.1002/bdra.23262.
-
7.
Ruangritnamchai C, Bunjapamai W, Pongpanich B. Pulse oximetry screening for clinically unrecognized critical congenital heart disease in the newborns. Images Paediatr Cardiol. 2007;9(1):10-5. [PubMed ID: 22368668]. [PubMed Central ID: PMC3232575].
-
8.
Acharya G, Sitras V, Maltau JM, Dahl LB, Kaaresen PI, Hanssen TA, et al. Major congenital heart disease in Northern Norway: shortcomings of pre- and postnatal diagnosis. Acta Obstet Gynecol Scand. 2004;83(12):1124-9. [PubMed ID: 15548143]. https://doi.org/10.1111/j.0001-6349.2004.00404.x.
-
9.
Bradshaw EA, Cuzzi S, Kiernan SC, Nagel N, Becker JA, Martin GR. Feasibility of implementing pulse oximetry screening for congenital heart disease in a community hospital. J Perinatol. 2012;32(9):710-5. [PubMed ID: 22282131]. [PubMed Central ID: PMC3432220]. https://doi.org/10.1038/jp.2011.179.
-
10.
Plana MN, Zamora J, Suresh G, Fernandez-Pineda L, Thangaratinam S, Ewer AK. Pulse oximetry screening for critical congenital heart defects. Cochrane Database Syst Rev. 2018;3. CD011912. [PubMed ID: 29494750]. [PubMed Central ID: PMC6494396]. https://doi.org/10.1002/14651858.CD011912.pub2.
-
11.
Eckersley L, Sadler L, Parry E, Finucane K, Gentles TL. Timing of diagnosis affects mortality in critical congenital heart disease. Arch Dis Child. 2016;101(6):516-20. [PubMed ID: 26130379]. https://doi.org/10.1136/archdischild-2014-307691.
-
12.
Ewer AK, Furmston AT, Middleton LJ, Deeks JJ, Daniels JP, Pattison HM, et al. Pulse oximetry as a screening test for congenital heart defects in newborn infants: a test accuracy study with evaluation of acceptability and cost-effectiveness. Health Technol Assess. 2012;16(2):v-xiii. 1-184. [PubMed ID: 22284744]. https://doi.org/10.3310/hta16020.
-
13.
Kardasevic M, Jovanovic I, Samardzic JP. Modern Strategy for Identification of Congenital Heart Defects in the Neonatal Period. Med Arch. 2016;70(5):384-8. [PubMed ID: 27994302]. [PubMed Central ID: PMC5136435]. https://doi.org/10.5455/medarh.2016.70.384-388.
-
14.
Ewer AK, Middleton LJ, Furmston AT, Bhoyar A, Daniels JP, Thangaratinam S, et al. Pulse oximetry screening for congenital heart defects in newborn infants (PulseOx): a test accuracy study. Lancet. 2011;378(9793):785-94. [PubMed ID: 21820732]. https://doi.org/10.1016/S0140-6736(11)60753-8.
-
15.
Movahedian AH, Mosayebi Z, Sagheb S. Evaluation of Pulse Oximetry in the Early Detection of Cyanotic Congenital Heart Disease in Newborns. J Tehran Heart Cent. 2016;11(2):73-8. [PubMed ID: 27928258]. [PubMed Central ID: PMC5027164].
-
16.
Mahle WT, Newburger JW, Matherne GP, Smith FC, Hoke TR, Koppel R, et al. Role of pulse oximetry in examining newborns for congenital heart disease: a scientific statement from the American Heart Association and American Academy of Pediatrics. Circulation. 2009;120(5):447-58. [PubMed ID: 19581492]. https://doi.org/10.1161/CIRCULATIONAHA.109.192576.
-
17.
Minocha P, Agarwal A, Jivani N, Swaminathan S. Evaluation of Neonates With Suspected Congenital Heart Disease: A New Cost-Effective Algorithm. Clin Pediatr (Phila). 2018;57(13):1541-8. [PubMed ID: 30094999]. https://doi.org/10.1177/0009922818793341.
-
18.
Thangaratinam S, Brown K, Zamora J, Khan KS, Ewer AK. Pulse oximetry screening for critical congenital heart defects in asymptomatic newborn babies: a systematic review and meta-analysis. Lancet. 2012;379(9835):2459-64. [PubMed ID: 22554860]. https://doi.org/10.1016/S0140-6736(12)60107-X.
-
19.
Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)--explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health. 2013;16(2):231-50. [PubMed ID: 23538175]. https://doi.org/10.1016/j.jval.2013.02.002.
-
20.
Griebsch I, Knowles RL, Brown J, Bull C, Wren C, Dezateux CA. Comparing the clinical and economic effects of clinical examination, pulse oximetry, and echocardiography in newborn screening for congenital heart defects: a probabilistic cost-effectiveness model and value of information analysis. Int J Technol Assess Health Care. 2007;23(2):192-204. [PubMed ID: 17493305]. https://doi.org/10.1017/S0266462307070304.
-
21.
Roberts TE, Barton PM, Auguste PE, Middleton LJ, Furmston AT, Ewer AK. Pulse oximetry as a screening test for congenital heart defects in newborn infants: a cost-effectiveness analysis. Arch Dis Child. 2012;97(3):221-6. [PubMed ID: 22247242]. https://doi.org/10.1136/archdischild-2011-300564.
-
22.
Peterson C, Grosse SD, Oster ME, Olney RS, Cassell CH. Cost-effectiveness of routine screening for critical congenital heart disease in US newborns. Pediatrics. 2013;132(3):e595-603. [PubMed ID: 23918890]. [PubMed Central ID: PMC4470475]. https://doi.org/10.1542/peds.2013-0332.
-
23.
Tobe RG, Martin GR, Li F, Moriichi A, Wu B, Mori R. Cost-effectiveness analysis of neonatal screening of critical congenital heart defects in China. Medicine (Baltimore). 2017;96(46). e8683. [PubMed ID: 29145300]. [PubMed Central ID: PMC5704845]. https://doi.org/10.1097/MD.0000000000008683.
-
24.
Narayen IC, Te Pas AB, Blom NA, van den Akker-van Marle ME. Cost-effectiveness analysis of pulse oximetry screening for critical congenital heart defects following homebirth and early discharge. Eur J Pediatr. 2019;178(1):97-103. [PubMed ID: 30334077]. [PubMed Central ID: PMC6311198]. https://doi.org/10.1007/s00431-018-3268-x.
-
25.
Mukerji A, Shafey A, Jain A, Cohen E, Shah PS, Sander B, et al. Pulse oximetry screening for critical congenital heart defects in Ontario, Canada: a cost-effectiveness analysis. Can J Public Health. 2020;111(5):804-11. [PubMed ID: 31907759]. https://doi.org/10.17269/s41997-019-00280-7.
-
26.
Londono Trujillo D, Sandoval Reyes NF, Taborda Restrepo A, Chamorro Velasquez CL, Dominguez Torres MT, Romero Ducuara SV, et al. Cost-effectiveness analysis of newborn pulse oximetry screening to detect critical congenital heart disease in Colombia. Cost Eff Resour Alloc. 2019;17:11. [PubMed ID: 31285695]. [PubMed Central ID: PMC6591944]. https://doi.org/10.1186/s12962-019-0179-2.
-
27.
Fixler DE, Xu P, Nembhard WN, Ethen MK, Canfield MA. Age at referral and mortality from critical congenital heart disease. Pediatrics. 2014;134(1):e98-105. [PubMed ID: 24982105]. https://doi.org/10.1542/peds.2013-2895.
-
28.
Brown KL, Ridout DA, Hoskote A, Verhulst L, Ricci M, Bull C. Delayed diagnosis of congenital heart disease worsens preoperative condition and outcome of surgery in neonates. Heart. 2006;92(9):1298-302. [PubMed ID: 16449514]. [PubMed Central ID: PMC1861169]. https://doi.org/10.1136/hrt.2005.078097.
-
29.
Abouk R, Grosse SD, Ailes EC, Oster ME. Association of US State Implementation of Newborn Screening Policies for Critical Congenital Heart Disease With Early Infant Cardiac Deaths. JAMA. 2017;318(21):2111-8. [PubMed ID: 29209720]. [PubMed Central ID: PMC5770276]. https://doi.org/10.1001/jama.2017.17627.