Abstract
Background:
Acute gastroenteritis is one of the most common diseases in children with a high rate of nausea and vomiting. Drugs such as ondansetron are used to treat vomiting.Objectives:
The aim of the study was to compare the success rate of oral vs intramuscular ondansetron to reduce vomiting in children with acute gastroenteritis.Methods:
A single-blind randomized clinical trial study was conducted on 100 children with acute gastroenteritis. Two groups of patients were created; one group received an intramuscular injection of ondansetron and the other received oral ondansetron. The vomiting rate, hospitalization, and side effects were evaluated 30 minutes, 4, and 48 hours after drug administration.Results:
Fifty-nine (59%) participants were boys. The mean age of the patients was 3.07 ± 2.20 years. There were no significant differences between the groups in terms of age, weight, and rate of vomiting before the treatment was launched. No significant difference between the drug administration route and the outcome of vomiting during the first half, 4, and 48 hours after receiving the drug was found either.Conclusions:
The study showed that the success rate of oral ondansetron did not vary significantly compared to intramuscular injection in terms of reducing the vomiting rate in children with acute gastroenteritis.Keywords
Gastroenteritis Children Injection Intramuscular Ondansetron Vomiting
1. Background
Acute gastroenteritis is one of the most common diseases and common causes of hospitalization in children (1). The most common cause are viral infections (2). In developing countries, it is the third leading cause of death in children under the age of five (3). Nausea and vomiting are very unpleasant conditions and cause difficulties for oral fluid therapy. On the other hand, attention to the state of hydration and electrolyte balance in acute gastroenteritis patients is a basic step for treatment. Therefore, the use of an antiemetic agent to control nausea and vomiting can increase the success of oral rehydration therapy (ORT) (4, 5), leading to lower hospitalization rates (6). Various medications are being used to treat vomiting, including Metoclopramide, Dimenhydrinate, Promethazine, and Domperidone (7). Ondansetron, selective antagonist of the 5HT-3 serotonin receptor, is a high-potent anti-emetic drug (8). Ondansetron has limited side effects compared to other anti-emetic drugs. The most common side effects are headache and dizziness that are mild and often self-limited. Tachycardia is another complication, usually mild and brief especially in children without underlying cardiac disease (8). Fortunately, prescription of a single dose of ondansetron for prevention of nausea and vomiting in patients with acute gastroenteritis without any dangerous underlying disease is safe and requires no electrocardiogram or electrolyte tests (6). Recent studies have shown that using ondansetron at the pediatric emergency department, either oral or injected, could reduce the need for hospital admission (9, 10). Although oral ondansetron is well tolerated by children (11), some patients need intramuscular injection (12, 13). In addition, intravenous injection of ondansetron is commonly used in children with cancer, as an anti-emetic drug after chemotherapy, radiotherapy and post-operative because of its few side effects (8, 14, 15). A study in California (2011) has found that ondansetron through any route of administration (oral, intravenous, and intramuscular injection) can reduce vomiting frequency (16). It is usually recommended to use non-invasive procedures (i.e., oral vs injection) for pediatric treatments, diagnostic procedures, or clinical measurements.
2. Objectives
The present study aimed to compare the effectiveness of oral and intramuscular injection of ondansetron to reduce vomiting in children with acute gastroenteritis and evaluate the rate of hospitalization and need for intravenous treatment, for the first time in Iran.
3. Methods
3.1. Study Design
We conducted a single-blind randomized clinical trial study on children with acute gastroenteritis at the emergency department of Bahrami Children's Hospital, Tehran, Iran, from April to September 2018.
3.2. Inclusion Criteria
Children, aged 1 to 10 years, suffering from acute gastroenteritis with moderate dehydration, based on clinical signs and symptoms, were recruited to the survey. The symptoms included tachycardia, decreased urine output, irritability/lethargy, sunken eyes, depressed fontanel, tears reduction, dry mouth mucous membranes, prolongation of skin turgor, delayed capillary refill (> 1.5 sec), cold and pale lower extremities. Their illness has started in the last 24 hours with vomiting (oral intolerance).
3.3. Exclusion Criteria
1- using any anti-emetic drug, 2- children with any chronic disease or alarm sign (i.e., headache, abdominal distention, severe dehydration, or shock), 3- severe diarrhea with more than one episode of defecation per hour, 4- a history of allergy to 5-HT3 receptor antagonists, 5- dysentery, 6- surgical problems, such as intussusception, 7- who has taken other medications.
3.4. Sample Size
According to the following two ratios for analytical studies formula the sample size was obtained (n = 92), which increased to 100 in order to increase the study capability. The study power was considered to be 80% and type one error less than 5%.
α = 0.05 (95% confidence level)
β = 20% (80% power)
Acceptable error rate = 0.2
Z1-α/2 = 1.96
Z1-β = 1.28
d = 0.2
3.5. Intervention
One hundred patients with acute gastroenteritis and moderate dehydration were recruited to the survey. Their illness had begun within the last 24 hours and had not responded to oral therapy. The study criteria for eligible patients were checked by pediatrician at the hospital emergency room. For eligible participants a structured questionnaire was completed, including demographic information (i.e., name, age, sex, and weight), emesis frequency/duration, and vomiting episodes before the admission. In addition, the patient’s contact information was gathered for the future follow-up. Prior to the intervention, a person who was not a researcher in the present study made a uniform package for both ondansetron syrup and ondansetron vials. The study’s researcher didn't know the contents of the packages. Each participant had the same chance to receive each treatment pack by a simple randomized sampling method. Thus, the half of patients allocated for syrup treatment and other half for the ondansetron vial (50 children in each group).
The injection group received an intramuscular ondansetron (Tehran Chemie Pharmaceutical Co, Iran) in the maximus gluteus muscle, with a dose of 0.2 mg/kg maximum 4 mg (17). The oral group received ondansetron with the same dose. Half an hour after receiving the medication, oral rehydration therapy (ORT) was begun with ORS at low volume (5 cc) and in short intervals (every 5 minutes). Those children who did not vomit for thirty minutes after receiving ondansetron were monitored in outpatient clinic for four hours. They were evaluated for vomiting relapse and common complications of ondansetron, including headache, tachycardia, and exacerbation of diarrhea. In children under 4 years, restlessness following medication was considered a side effect of the drug, such as headache. The upper limits of normal heart rate in children vary according to age and sex. In children aged 1-10 years it ranged 110 - 130 beats/min (18). The baseline heart rate of patients was compared with the heart rate after drug administration, and it was considered a complication of the drug if the rate increased. In cases where the exacerbation of diarrhea was seen along with the improvement of other symptoms, it was considered as a side effect of the drug.
After the monitoring, those patients who had oral tolerance were discharged. The discharge criteria included: full tolerance of ORT and the relative improvement in dehydration during monitoring. The participants were followed up by telephone up to 48 hours after discharge. Children who did not tolerate oral therapy were hospitalized and received intravenous treatment. Evaluation of response to the medication at the time of 0.5, 4 and 48 hours after starting treatment was performed. Since the onset of oral effect is 30 minutes its peak effect is 4 hours (19) and the duration of vomiting in acute GE is on average up to 48 hours (20). The medication was prescribed in only one dose, and no drugs were recommended for use at home. Therefore, patients who received additional doses or other medications were excluded from the study.
3.6. Ethical Consideration
We explained the basics of research, complications, and potential risks of each method to the parents. Confidentiality of their information was guaranteed. Then, the parents signed the study written informed consent. This study was approved by the Ethics Committee of the Tehran University of Medical Sciences (approval ethics code: IR.TUMS.MEDICINE.REC.1396.2713) and was registered at the Iranian Registry of Clinical Trials (approval RCT code: IRCT2017082118971N5).
3.7. Statistical Analysis
The information was collected in a separate questionnaire and analyzed by SPSS statistical software (version 24) using chi-square (for sex, vomiting, evaluation times) and Mann-Whitney tests. The Kolmogorov-Smirnov test was used to investigate the normal distribution of quantitative data. All variables were significant in this test, without normal distribution. Therefore, nonparametric tests were used.
4. Results
One hundred children - two groups of fifty - with acute gastroenteritis, vomiting and oral nutrition intolerance, were studied. Fifty-nine (59%) were male, 29 (58%) belonged to the injection group and 30 (60%) to the oral group. There was no significant difference in gender between the groups (P-value = 0.839). The mean age and weight were 3.07 ± 2.20 years and 14.17 ± 5.25 kg, respectively. The frequency of vomiting before admission to the emergency department was 4.45 ± 2.42 times per day. In average, emesis had started 5.71 ± 3.38 hours before the medical consultation. Table 1 summarizes the frequency of the quantitative data. There were no significant differences between the groups in terms of the age (years), weight (kilograms), and frequency of vomiting (times per day) before admission. Nine (9%) patients had vomiting during the first 30 minutes after receiving the drug, five (10%) children belonged to the injection group and four (8%) to the oral. There were no significant differences in terms of route administration and vomiting. There was no significant difference in vomiting frequency in the first 30 minutes pertaining to age and weight. Three (3%) patients had vomiting within 4 hours after treatment; two (4%) were in the injection group and one (2%) in the oral group. There was no significant relationship between the drug administration route and vomiting within 4 hours. Also, in both groups, there was no significant correlation between vomiting in the first 4 hours and age or weight (P-value > 0.05). Seven (7%) patients had vomiting within 48 hours after drug administration, of which five (10%) belonged to the injection group and two (4%) to the oral. There was no significant relationship between the drug administration route and vomiting within 48 hours (P-value = 0.436). The age and weight of the children had a significant correlation with the incidence of vomiting in the first 48 hours, so that the age and weight in the group who had emesis within 48 hours after the intervention was significantly lower than the group who did not vomit (P-value = 0.008, P-value = 0.012, respectively). Table 2 summarizes vomiting rate following the drug administration method based on children’s gender (Figure 1).
Descriptive Statistics of the Quantitative Data
| Variable | Mean ± SD | Maximum | Minimum | Median |
|---|---|---|---|---|
| Age (y) | 3.07 ± 2.20 | 9.00 | 1.00 | 2.00 |
| Weight (kg) | 14.17 ± 5.03 | 30.00 | 6.20 | 12.50 |
| Number of hours of emesis before referral (h) | 5.71 ± 3.38 | 12.00 | 1.00 | 5.00 |
| Number of vomiting before referral (p/d) | 4.45 ± 2.42 | 12.00 | 1.00 | 4.00 |
Frequency of Vomiting and Oral Rehydration Therapy (ORT) Tolerance After Administration of Ondansetron Based on Gender and Administration Method a
| Administration Route | ORT Tolerance | Vomiting Within 30 Minutes | Vomiting During 4 Hours | Vomiting During 48 Hours |
|---|---|---|---|---|
| Injection | ||||
| Male | 21 (72.4) | 3 (10.3) | 2 (6.9) | 3 (10.3) |
| Female | 17 (76.0) | 2 (9.5) | 0 (0.0) | 2 (9.5) |
| Total | 38 (82.9) | 5 (10.3) | 2 (4.0) | 5 (10.3) |
| Oral | ||||
| Male | 26 (86.7) | 4 (13.3) | 0 (0.0) | 0 (0.0) |
| Female | 17 (85.0) | 0 (0.0) | 1 (5.0) | 2 (10.0) |
| Total | 43 (86.0) | 4 (8.0) | 1 (2.0) | 2 (4.0) |
| Total regardless of the gender | 81 (81.0) | 9 (9.0) | 3 (3.0) | 7 (7.0) |
consort flow diagram of study groups.
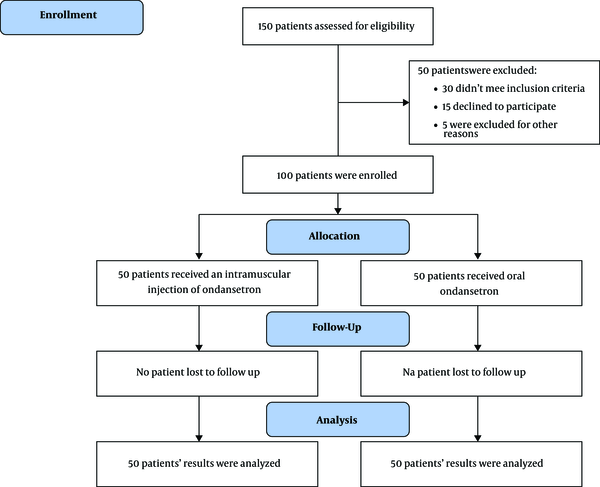
Totally, eighty-one (81%) patients tolerated ORT, 38 (76.0%) of them belonged to the injection group and 43 (86.8%) to the oral. This difference was not statistically significant. There was no relationship between age and gender with ORT tolerance. Table 2 shows the frequency of ORT tolerance based on gender and method of administration.
Twelve (24%) patients of the injection group and seven of the oral (14%) were hospitalized and intravenous fluid therapy initiated (P-value = 0.202). There was no relationship between age and gender with the need for hospitalization.
Four patients suffered from mild headache after receiving the drug, which improved spontaneously. Tachycardia did not develop in any child. During the monitoring period, the heart rate was within the normal range for age. Two patients developed diarrhea after receiving the drug. Table 3 summarizes the drug side effects. There were no significant difference between the drug administration route and these side effects (headache, tachycardia, the exacerbation of diarrhea). Similarly, there was no relationship between age and gender with these side effects of the drug (P-value > 0.05).
Frequency of Drug Side Effects in the Administration Methods of Ondansetron in Children with Vomiting a
| Side Effect | Total | Injection | Oral | P-Value |
|---|---|---|---|---|
| Headache | 4 (4) | 2 (4) | 2 (4) | 1.000 |
| Tachycardia | 0 (0) | 0 (0) | 0 (0) | --- |
| Diarrhea | 2 (2) | 0 (0) | 2 (2) | 0.495 |
5. Discussion
The aim of this study was to compare the effectiveness of oral and intramuscular injections of ondansetron in reducing vomiting in children with acute gastroenteritis. According to the results, the effectiveness of both methods in controlling vomiting has been equal. Vomiting due to gastroenteritis causes difficulty for oral rehydration in children. ORT is very important in the treatment of gastroenteritis (21). Based on the results of previous studies, vomiting due to acute gastroenteritis in 60% of children aged 1 to 6 years is treated with ORT (22). If emesis persists, the use of an anti-emetic agent can lead to oral tolerance of ORT and reduce the need for hospitalization (4, 5). Tomasik et al compared the effect of intravenous ondansetron with placebo and observed that ondansetron was superior in reducing vomiting (23). Unfortunately, there is not a guideline for using antiemetic drugs to treat gastroenteritis globally. Danewa et al showed that the group receiving ondansetron had a greater reduction in vomiting than those receiving placebo (31% versus 62%), but, there was no significant relationship between groups in terms of the need for hospitalization (24). Similarly; a meta-analysis study shows that ondansetron is effective to reduce vomiting in children with gastroenteritis. However, there is not enough evidence for the routine use of it at the emergency room (25). Several studies demonstrated that oral ondansetron is effective in reducing vomiting and the need for hospitalization compared to placebo and other antiemetic drugs (5, 8, 19, 23, 26, 27). In our study, administration of ondansetron through both oral and intramuscular routes during the first 4 hours significantly reduced the vomiting and improved ORT tolerance, which was consistent with other studies (22, 26, 27). In our experience, ondansetron was effective in reducing emesis 48 hours after administration (only 7% of the children vomited), which was consistent with Freedman’s results (26). We did not find significant differences between the groups in terms of side effects. This finding was similar to the results of Golshekan (27). In Freedman's study, diarrhea was more frequent in the ondansetron-receiving group than in the placebo group (1.4 vs 0.5, P-value < 0.001) (26). Some authors reported that exacerbation of diarrhea was the most important complication (8, 23, 28). We found that only two (2%) children had diarrhea exacerbation within 48 hours. Although acute gastroenteritis is considered a self-limited disease, the use of ondansetron through both routes - oral and injection - reduces the need of hospitalization and intravenous fluid therapy. Therefore, it is reasonable to use this drug due to its low side effects compared with other anti-emetics. This drug can reduce the need of hospital admission, the duration of observation time at the emergency department and the treatment cost (22).
Our study was designed to evaluate non-invasive treatments in children, and showed that both methods (oral and injection) have the same effect. Therefore, it is reasonable to recommend the use of non-invasive methods (oral) in the treatment of pediatric vomiting. Our study was a randomized clinical trial during which we encountered some problems and limitations, therefore, studies with larger sample size should be conducted to achieve more conclusive results. Study limitations appeared during the follow up after discharge. The information of parents or care-takers was the source of evaluation, and this increased the risk of error. Some parents refused to use oral medication, and others did not cooperate to complete the treatment.
5.1. Conclusion
The present study suggests that there is no significant difference between the therapeutic routes (oral or injection) in terms of the rate of hospitalization, reduction of emesis, ORT tolerance, and side effects. Both treatment methods can be used to reduce vomiting in children with acute gastroenteritis. According to our findings, in children with acute gastroenteritis, the use of oral ondansetron is better because it is non-invasive.
Acknowledgements
References
-
1.
Guarino A, Ashkenazi S, Gendrel D, Lo Vecchio A, Shamir R, Szajewska H, et al. European society for pediatric gastroenterology, hepatology, and nutrition/european society for pediatric infectious diseases evidence-based guidelines for the management of acute gastroenteritis in children in europe: Update 2014. J Pediatr Gastroenterol Nutr. 2014;59(1):132-52. [PubMed ID: 24739189]. https://doi.org/10.1097/MPG.0000000000000375.
-
2.
King CK, Glass R, Bresee JS, Duggan C; Centers for Disease Control and Prevention. Managing acute gastroenteritis among children: oral rehydration, maintenance, and nutritional therapy. MMWR Recomm Rep. 2003;52(RR-16):1-16. [PubMed ID: 14627948].
-
3.
Florez ID, Al-Khalifah R, Sierra JM, Granados CM, Yepes-Nunez JJ, Cuello-Garcia C, et al. The effectiveness and safety of treatments used for acute diarrhea and acute gastroenteritis in children: protocol for a systematic review and network meta-analysis. Syst Rev. 2016;5:14. [PubMed ID: 26818403]. [PubMed Central ID: PMC4728803]. https://doi.org/10.1186/s13643-016-0186-8.
-
4.
Reeves JJ, Shannon MW, Fleisher GR. Ondansetron decreases vomiting associated with acute gastroenteritis: a randomized, controlled trial. Pediatrics. 2002;109(4). e62. [PubMed ID: 11927735]. https://doi.org/10.1542/peds.109.4.e62.
-
5.
Roslund G, Hepps TS, McQuillen KK. The role of oral ondansetron in children with vomiting as a result of acute gastritis/gastroenteritis who have failed oral rehydration therapy: a randomized controlled trial. Ann Emerg Med. 2008;52(1):22-29 e6. [PubMed ID: 18006189]. https://doi.org/10.1016/j.annemergmed.2007.09.010.
-
6.
Schnadower D, Finkelstein Y, Freedman SB. Ondansetron and probiotics in the management of pediatric acute gastroenteritis in developed countries. Curr Opin Gastroenterol. 2015;31(1):1-6. [PubMed ID: 25333367]. https://doi.org/10.1097/MOG.0000000000000132.
-
7.
Manteuffel J. Use of antiemetics in children with acute gastroenteritis: Are they safe and effective? J Emerg Trauma Shock. 2009;2(1):3-5. [PubMed ID: 19561947]. [PubMed Central ID: PMC2700583]. https://doi.org/10.4103/0974-2700.44674.
-
8.
Cheng A. Emergency department use of oral ondansetron for acute gastroenteritis-related vomiting in infants and children. Paediatr Child Health. 2011;16(3):177-82. [PubMed ID: 22379383]. [PubMed Central ID: PMC3077311]. https://doi.org/10.1093/pch/16.3.177.
-
9.
Freedman SB, Ali S, Oleszczuk M, Gouin S, Hartling L. Treatment of acute gastroenteritis in children: an overview of systematic reviews of interventions commonly used in developed countries. Evid Based Child Health. 2013;8(4):1123-37. [PubMed ID: 23877938]. https://doi.org/10.1002/ebch.1932.
-
10.
Thompson GC, Morrison EL, Chaulk D, Wobma H, Kwong S, Johnson DW. Ondansetron oral dissolve tab vs. Oral solution in children presenting to the emergency department with gastroenteritis. J Emerg Med. 2016;51(5):491-7. [PubMed ID: 27624509]. https://doi.org/10.1016/j.jemermed.2016.06.051.
-
11.
Howard S. Archimedes. Question 1. Does oral ondansetron reduce vomiting and the need for intravenous fluids and hospital admission in children presenting with vomiting secondary to gastroenteritis? Arch Dis Child. 2010;95(11):945-7. [PubMed ID: 20980278]. https://doi.org/10.1136/adc.2010.198937.
-
12.
Colletti JE, Brown KM, Sharieff GQ, Barata IA, Ishimine P, Acep Pediatric Emergency Medicine Committee. The management of children with gastroenteritis and dehydration in the emergency department. J Emerg Med. 2010;38(5):686-98. [PubMed ID: 19345549]. https://doi.org/10.1016/j.jemermed.2008.06.015.
-
13.
Freedman SB, Pasichnyk D, Black KJ, Fitzpatrick E, Gouin S, Milne A, et al. Gastroenteritis therapies in developed countries: Systematic review and meta-analysis. PLoS One. 2015;10(6). e0128754. [PubMed ID: 26075617]. [PubMed Central ID: PMC4468143]. https://doi.org/10.1371/journal.pone.0128754.
-
14.
Herrstedt J, Roila F, Warr D, Celio L, Navari RM, Hesketh PJ, et al. 2016 updated MASCC/ESMO consensus recommendations: Prevention of nausea and vomiting following high emetic risk chemotherapy. Support Care Cancer. 2017;25(1):277-88. [PubMed ID: 27443154]. https://doi.org/10.1007/s00520-016-3313-0.
-
15.
Dupuis LL, Boodhan S, Holdsworth M, Robinson PD, Hain R, Portwine C, et al. Guideline for the prevention of acute nausea and vomiting due to antineoplastic medication in pediatric cancer patients. Pediatr Blood Cancer. 2013;60(7):1073-82. [PubMed ID: 23512831]. https://doi.org/10.1002/pbc.24508.
-
16.
Salvucci AA, Squire B, Burdick M, Luoto M, Brazzel D, Vaezazizi R. Ondansetron is safe and effective for prehospital treatment of nausea and vomiting by paramedics. Prehosp Emerg Care. 2011;15(1):34-8. [PubMed ID: 21091329]. https://doi.org/10.3109/10903127.2010.519822.
-
17.
Greenbaum LA. Dificit therapy. In: Kliengman RM, Stanton BF, Geme JWS, Schor NF, editors. Nelson Text Book of Pediatrics. 20th ed. Philadelphia: Saunders Elsevier; 2016. p. 388-91.
-
18.
Bernstein D. History and physical examination. In: Kliengman RM, Stanton BF, Geme JWS, Schor NF, editors. Nelson Text book of pediatrics. 20th ed. Philadelphia: Saunders Elsevier; 2016. p. 2163-9.
-
19.
Roila F, Del Favero A. Ondansetron clinical pharmacokinetics. Clin Pharmacokinet. 1995;29(2):95-109. [PubMed ID: 7586904]. https://doi.org/10.2165/00003088-199529020-00004.
-
20.
Bass DM. Rotaviruses, caliciviruses, and astroviruses. In: Kliengman RM, Stanton BF, Geme JWS, Schor NF, editors. Nelson Text book of pediatrics. 20th ed. Philadelphia: Saunders Elsevier; 2016. p. 1616-8.
-
21.
Albano F, Bruzzese E, Spagnuolo MI, De Marco G. Antiemetics for children with gastroenteritis: off-label but still on in clinical practice. J Pediatr Gastroenterol Nutr. 2006;43(3):402-4. [PubMed ID: 16954969]. https://doi.org/10.1097/01.mpg.0000232571.56397.0d.
-
22.
Marchetti F, Bonati M, Maestro A, Zanon D, Rovere F, Arrighini A, et al. Oral Ondansetron versus Domperidone for Acute Gastroenteritis in Pediatric Emergency Departments: Multicenter Double Blind Randomized Controlled Trial. PLoS One. 2016;11(11). e0165441. [PubMed ID: 27880811]. [PubMed Central ID: PMC5120790]. https://doi.org/10.1371/journal.pone.0165441.
-
23.
Tomasik E, Ziolkowska E, Kolodziej M, Szajewska H. Systematic review with meta-analysis: ondansetron for vomiting in children with acute gastroenteritis. Aliment Pharmacol Ther. 2016;44(5):438-46. [PubMed ID: 27401959]. https://doi.org/10.1111/apt.13728.
-
24.
Danewa AS, Shah D, Batra P, Bhattacharya SK, Gupta P. Oral ondansetron in management of dehydrating diarrhea with vomiting in children aged 3 months to 5 years: A randomized controlled trial. J Pediatr. 2016;169:105-9 e3. [PubMed ID: 26654135]. https://doi.org/10.1016/j.jpeds.2015.10.006.
-
25.
Fedorowicz Z, Alhashimi D, Alhashimi H. Meta-analysis: ondansetron for vomiting in acute gastroenteritis in children. Aliment Pharmacol Ther. 2007;26(7):1086. author reply 1087. [PubMed ID: 17877518]. https://doi.org/10.1111/j.1365-2036.2007.03441.x.
-
26.
Freedman SB, Adler M, Seshadri R, Powell EC. Oral ondansetron for gastroenteritis in a pediatric emergency department. N Engl J Med. 2006;354(16):1698-705. [PubMed ID: 16625009]. https://doi.org/10.1056/NEJMoa055119.
-
27.
Golshekan K, Badeli H, Rezaieian S, Mohammadpour H, Hassanzadehrad A. Effect of oral ondansetron on decreasing the vomiting associated with acute gastroenteritis in Iranian children. Iran J Pediatr. 2013;23(5):557-63. [PubMed ID: 24800017]. [PubMed Central ID: PMC4006506].
-
28.
Lalani N, Gaco D. Ondansetron for gastroenteritis in children and adolescents. Am Fam Physician. 2015;91(7):Online. [PubMed ID: 25884755].