1. Background
Acute appendicitis is a common acute abdominal disease in pediatric surgery (1), and delayed diagnosis and treatment can easily lead to complications such as appendix perforation and sepsis (2, 3). The pathogenesis of the disease is still not fully understood, and obstruction of the appendiceal cavity and bacterial infection are considered to be the main causes of the disease (4), among which Escherichia coli and Pseudomonas aeruginosa are the common pathogens (5, 6). Due to the particularity of pediatric patients, the diversity of antibacterial drugs and the increase of multi-drug resistance of bacteria, the antibiotic treatment program for acute appendicitis in children becomes increasingly complex (7, 8). Therefore, the understanding of pathogenic bacteria and the drug resistance spectrumis is conducive to the optimization of the empirical antimicrobial treatment program and the improvement of the clinical treatment effect, can also reduce the use of unnecessary antimicrobial agents.
Studies have found that the number of P. aeruginosa isolated from the secretion of the appendix cavity of patients with acute appendicitis is positively correlated to the infection degree of the operation site, and the reason may be related to the lack of corresponding antibiotics in the primary treatment scheme (9, 10). Although imaging techniques (11, 12), inflammatory factors (13-17), some clinical biochemical indicators (18, 19) and some blood metabolites (20) have been able to diagnose acute appendicitis, the pathogenic bacteria and drug resistance spectrum in patients have not been determined by non-interventional methods.
2. Objectives
This study intends to establish a suitable non-interventional differential diagnosis model to determine whether the pathogenic bacteria in children are P. aeruginosa and if so, to determine its antibiotic resistance through statistical analysis of clinical blood biochemical indicators of children with acute appendicitis, and to screen out appropriate predictors.
3. Methods
3.1. Study Population
This retrospective study was approved by the Ethics Committee of Jiangxi Provincial Children’s Hospital (code: No.JXSETYY-YXKY-20190096). In this study, patients with acute appendicitis admitted to the general surgery department of Jiangxi children’s hospital from January 2015 to June 2019 and treated surgically were enrolled. A total of 153 patients were included in this study, aged 1 - 16 years, 99 males and 54 females. All samples were identified and tested by BD Phoenix, a microbial identification and drug sensitivity analysis system. The subjects’ appendiceal secretions or pus were taken for bacterial culture, among which 50 cases (group of control) of bacterial infection were not detected, and 103 cases of P. aeruginosa infection were detected. According to the resistance of P. aeruginosa to penicillin antibiotics, 72 cases were classified as drug resistant group (PAR+) and 31 cases as non-drug resistant group (PAR-). The clinical blood test parameters of all subjects are shown in Supplementary table 1 and the clinical information are summarized in Table 1.
| Group | Total Cases | Male | Female | Age, y | Hospital Stay, d | Appendix Perforated |
|---|---|---|---|---|---|---|
| Group of control | 50 | 35 | 15 | 6.51 ± 0.39 | 7.45 ± 0.33 | 2 |
| Pseudomonas aeruginosa (PRA+) | 72 | 46 | 16 | 6.80 ± 0.37 | 9.47 ± 0.37 | 60 |
| P. aeruginosa (PRA-) | 31 | 24 | 7 | 6.90 ± 0.53 | 9.83 ± 0.59 | 24 |
3.2. Statistical Analysis
All the data in this study were based on 71 clinical blood test indexes recorded in the original medical records of the subjects at admission. All the data were imported into SIMCA-P (version 12.0, Umetrics, Umea, Sweden) for partial lead squares discrimination analysis (PLS-DA) and SPSS23.0 (SPSS Inc., Chicago, IL, US) for Mann Whitney U-test, ROC analysis and odds ratio (OR) calculation.
4. Results
4.1. Analysis of Antibiotic Resistance of Pseudomonas aeruginosa in Abdominal Cavity of Children with Acute Appendicitis
Bacterial culture and antibiotic resistance experiments were conducted on exudates or pus in the appendiceal cavity of all subjects. A total of 103 strains of P. aeruginosa were isolated and showed resistance to 14 antibiotics (Table 2). Among them, 4 strains were sensitive to all types of antibiotics, 3 strains showed resistance to only one antibiotic, and 96 showed resistance to three or more antibiotics. Beta lactam antibiotic is the most common type of the 14 antibiotics (7/14), and 99 strains of P. aeruginosa showed resistance to beta-lactam antibiotic, with the resistance rate of 100.00% (99/99). The three drugs with the highest rate of individual antibiotic resistance were cefotaxime (93.20%, 96/103), cotrimoxazole (93.20%, 96/103), and tetracycline (93.20%, 96/103).
| No | Antibiotics | Pseudomonas aeruginosa (N= 103) | Total | Ratio of Resistance, % | |
|---|---|---|---|---|---|
| PAR- (N = 31) | PAR+ (N = 72) | ||||
| 1 | Ampicillin | 0 | 69 | 69 | 66.99 |
| 2 | Piperacillin | 0 | 3 | 3 | 2.91 |
| 3 | Cefepime | 0 | 2 | 2 | 1.94 |
| 4 | Cefotaxime | 24 | 72 | 96 | 93.20 |
| 5 | Ceftazidime | 0 | 2 | 2 | 1.94 |
| 6 | Cefazolin | 22 | 70 | 92 | 89.32 |
| 7 | Aztreonam | 2 | 7 | 9 | 8.74 |
| 8 | Cotrimoxazole | 24 | 72 | 96 | 93.20 |
| 9 | Tetracycline | 24 | 72 | 96 | 93.20 |
| 10 | Chloramghenicol | 23 | 72 | 95 | 92.23 |
| 11 | Gentamicin | 2 | 1 | 3 | 2.91 |
| 12 | Levofloxacin | 0 | 4 | 4 | 3.88 |
| 13 | Ciprofloxacin | 1 | 4 | 5 | 4.85 |
| 14 | Amikacin | 0 | 1 | 1 | 0.97 |
4.2. Analysis Results of Blood Indicators of all Subjects and the Establishment of Discriminant Model
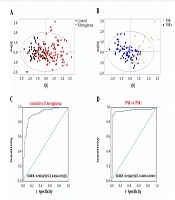
Subject data were imported into SIMCA-P for multivariate statistical analysis, and the results are shown in Figure 1A and B. Control/P. averuginosa and PAR+/PAR- were distributed in different areas of the figure, indicating significant pattern differences between Control/P. averuginosa and PAR+/PAR- (21). Control/P. averuginosa and PAR+/PAR- showed significant differences in 33 and 20 variables, respectively (Appendix 1 in Supplementary File). Among them, 7 variables (ALB, IDBL, GGT, CRP, P, FFA, and CFbg) showed significant differences among each group (Table 3).
| Factors | Control | Pseudomonas aeruginosa | P1 | P2 | |
|---|---|---|---|---|---|
| PAR- | PAR+ | ||||
| Num. | 50 | 31 | 72 | - | - |
| Male | 30 | 24 | 45 | 3.54E-01 | 1.65E-01 |
| Age, y | 6.51 ± 0.39 | 6.9 ± 0.53 | 6.80 ± 0.37 | 7.28E-02 | 5.99E-01 |
| ALB, g/L | 47.42 ± 0.42 | 45.43 ± 0.77 | 44.11 ± 0.42 | 8.97E-06 | 4.57E-02 |
| IDBL, µmol/L | 6.72 ± 0.34 | 10.18 ± 0.87 | 8.26 ± 0.47 | 2.35E-03 | 3.34E-02 |
| CRP, mg/L | 18.56 ± 3.58 | 67.31 ± 11.16 | 93.80 ± 7.87 | 1.45E-10 | 3.15E-02 |
| GGT, U/L | 8.52 ± 0.78 | 8.94 ± 0.96 | 12.04 ± 0.63 | 6.13E-05 | 4.00E-04 |
| P, mmol/L | 1.61 ± 0.04 | 1.39 ± 0.08 | 1.37 ± 0.06 | 2.37E-07 | 3.32E-05 |
| FFA, mmol/L | 0.90 ± 0.06 | 0.87 ± 0.09 | 1.22 ± 0.08 | 2.97E-04 | 3.35E-13 |
| CFbg, g/L | 2.79 ± 0.08 | 3.98 ± 0.22 | 4.53 ± 0.19 | 2.92E-12 | 3.31E-02. |
Abbreviations: ALB, albumin; CRP, C-reactive protein; CFbg, fibrinogen; FFA, free fatty acid; GGT, glutamyltranspeptidase; IDBL, indirect bilirubin; P, phosphorus; P1, P-value of Control vs. P. aeruginosa; P2, P-value of PAR- vs. PAR+.
aValues are expressed as mean ± SEM.
To establish a suitable differential diagnosis model, the above seven variables were used as parameters for binary logistic regression analysis, and the maximum likelihood estimation forward method (forward Wald) was used for stepwise regression analysis.
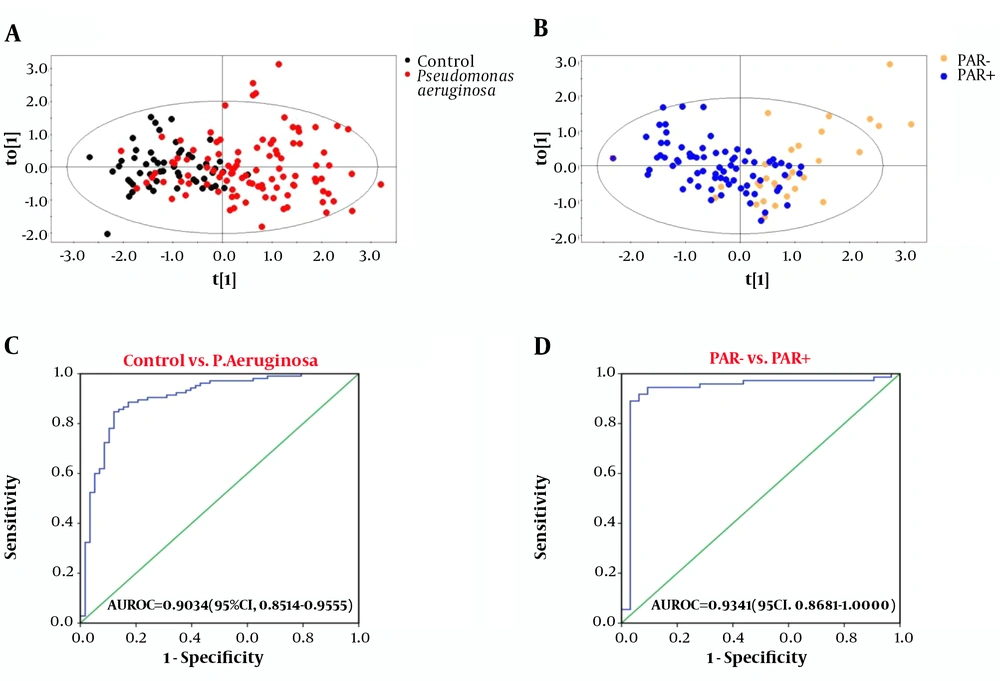
The results showed that control/P. aeruginosa and PAR+/PAR- could be effectively distinguished by combining GGT, P, FFA, and CFbg. For the control group and P. aeruginosa infected group, the AUROC value of the four indicators was 0.9034 (95% CI, 0.8514 - 0.9555), with a sensitivity and specificity of 84.76% and 87.93%, respectively (Figure 1C). For the group with or without penicillin antibiotics resistance, the AUROC value was 0.9341 (95% CI, 0.8681 - 1.0000), with a sensitivity and specificity of 89.04% and 96.88%, respectively (Figure 1D).
4.3. Predictive Analysis of Pseudomonas aeruginosa Infection and Drug Resistance in Children with Acute Appendicitis
Multiple logistic regression was used to analyze GGT, P, FFA, and CFbg variables, and the OR value of a single variable and the combination of the four variables were calculated respectively (Table 4). The results showed that the effect of the combination of four variables is significantly better than that of a single variable, and the OR value of control/P. aeruginosa and PAR+/PAR- were 655.9676 (95% CI, 109.9204 - 3914.5928) and 1115.8482 (95% CI, 121.2366 - 10270.1414), respectively. It suggests that the combination of GGT, P, FFA, and CFbg can be used to judge P. aeruginosa infection and penicillin antibiotics resistance in children with appendicitis to a certain extent.
| Control Vs. Pseudomonas aeruginosa | PAR- Vs. PAR+ | |||
|---|---|---|---|---|
| Factors | OR | 95% CI | OR | 95% CI |
| GGT | 1.1408 | 1.0386 - 1.2531 | 1.0468 | 0.9016 - 1.2154 |
| P | 0.0201 | 0.0025 - 0.1602 | 0.2001 | 0.0061 - 6.5142 |
| FFA | 5.3870 | 1.4451 - 20.0809 | 313.6216 | 90.1554 - 1778.4525 |
| CFbg | 3.4416 | 1.9576 - 6.0503 | 1.0174 | 0.6169 - 1.6781 |
| Conbination | 655.9676 | 109.9204 - 3914.5928 | 1115.8482 | 121.2366 - 10270.1414 |
5. Discussion
Bacterial infection and antibiotic resistance in patients with appendicitis are a difficult problem in today’s world, and how to develop effective treatment plans early is a challenge for clinicians (22). It is of great significance to understand the pathogenic bacteria and drug resistance spectrum of patients with appendicitis and use drugs in a targeted way to improve the clinical treatment effect, reduce the abuse of antibiotics and reduce the drug resistance rate of antibiotics. In this study, 103 children with acute appendicitis infected by P. aeruginosa were analyzed. It was found that the drug resistance rate of pathogenic bacteria to antibiotics was very high (96.12%, 99/103). The drug resistance rate of resistant bacteria to beta-lactam antibiotic reached 100.00% (99/99), and 96.97% (96/99) of resistant bacteria showed multiple drug resistance. Therefore, we should reduce the use of cefotaxime (93.20%), cefazolin (89.32%), cotrimoxazole (93.20%), tetracycline (93.20%) and chloromeganicol (92.23%) and other antibiotics with high drug resistance rate, and use highly sensitive antimicrobials such as cefepime (1.94%) and ceftazidime (1.94%).
In this study, for the first time, the pattern recognition analysis was conducted on the clinical indicators of acute appendicitis in children with negative bacterial culture and P. aeruginosa infection through multivariate statistical analysis, and found that the combination of GGT, P, FFA and CFbg could effectively distinguish control/P. aeruginosa and PAR+/PAR-. Compared with the control group, the contents of GGT, FFA, and CFbg in P. aeruginosa group increased, and increased with the increase of the P. aeruginosa resistance species, while the content of P in P. aeruginosa group decreased, and further decreased with the increase of the P. aeruginosa resistance species. These results suggest that GGT, P, FFA, and CFbg are closely related to acute appendicitis caused by P. aeruginosa infection and may be potential biomarkers. In this study, the children with negative bacterial culture might also be in the early stage of the disease. Because these children had used antibiotics, and the bacteria had been eliminated in the early stage, resulting in no pathogenic bacteria detected in the culture of appendix exudate or peritoneal pus taken out during the later operation.
With the occurrence of bacterial infection, the inflammatory response in the body is often further triggered (23). By analyzing CRP, an indicator of inflammation, we found that the degree of inflammation in children increased with the infection of P. aeruginosa and the increase of drug resistance. GGT is an indicator related to liver injury (24), and the increase in its content in this study may be related to the degree of inflammation caused by infection, or to the liver function damage caused by some substances (endotoxin, etc.) produced by bacteria. As bacteria proliferate in the abdominal cavity, it often affects the intestinal water salt balance and lipid metabolism. Therefore, the increase of FFA in the blood of patients in this study may be caused by the proliferation and release of P. aeruginosa, while the decrease of P may be caused by the metabolic consumption of P. aeruginosa. Extended spectrum β-lactamases (ESBLs) are produced in most patients with bacterial infection resistant to β-lactamases (25). Therefore, we speculate that the content difference of GGT, P, FFA, and CFbg may be related to the drug-resistant enzyme produced by bacteria, and the enzyme may have a certain impact on the body, but the specific mechanism needs further study.
5.1. Conclusions
This study found that children with acute appendicitis caused by P. aeruginosa showed a high resistance rate to antimicrobial agents. Through the combination of GGT, P, FFA, and CFbg, we could effectively determine whether the children with acute appendicitis were infected by P. aeruginosa and resistant to penicillin antibiotics, which could be used as a predictor, and is of great significance for guiding clinicians to choose treatment plans at an early stage.