1. Background
Bronchopulmonary dysplasia (BPD) is a chronic lung disease of preterm neonates. It is primarily characterized by persistent abnormalities of the lung structure and arrested lung development, e.g., impaired alveolar and vascular growth (1, 2). Despite advances in perinatal care, BPD remains a major cause of neonatal morbidity and mortality, and there are now a few therapeutic options for patients with BPD. Our previous study verified the therapeutic efficacy of bone marrow-derived mesenchymal stem cells (BMSCs) in the hyperoxia-induced BPD mouse model (3). BMSC delivered intraperitoneally can target injured lungs, improve pulmonary architecture, attenuate pulmonary fibrosis, and decrease pulmonary expression of a number of genes related to extracellular matrix (ECM) remodeling and fibrosis, e.g., transforming growth factor-β1 (TGF-β1), collagen 1a and tissue inhibitor of metalloproteinases-1, interleukin-1 (IL-1), and tumor necrosis factor-α (3, 4). This conclusion is consistent with the results of previous studies (5-11) and the data support that stem cell therapy is feasible.
MicroRNAs (miRNAs or miRs) are 21 - 25-nucleotides long non-coding RNAs involved in various biological processes, e.g., cell proliferation, cell death, stress resistance, and tumorigenesis (12, 13). Our previous study verified that microRNA-206 (miR-206) contributes to the pathogenesis of BPD by regulating the expression of fibronectin 1 (FN1). In the development of BPD, miR-206 is down-regulated in BPD patients and newborn mice, and targets FN1, an ECM glycoprotein involved in cell adhesion and migration, e.g., embryogenesis, wound healing, metastasis, and host defense (14).
2. Objectives
To study the interaction between BMSCs and miR-206 in the treatment of BPD, this study investigated the effect of BMSCs with up-regulation of miR-206 in a well-established hyperoxia-induced model of BPD by regulating BMSCs transfected with miR-206 intraperitoneally.
3. Methods
3.1. Cells
As previously described (15), BMSCs were obtained from the femoral bone marrow of mice (Kunming mice, 3 - 4-week-old males, purchased from the Chinese Academy of Sciences, Beijing, China). The analysis of cell surface immunophenotype suggested that the BMSC population was positive for cluster of differentiation 105 (CD105) and CD106 but negative for CD34 and CD45.
3.2. Plasmid
The plasmids of mmu-miR-206 (miR-206) and miRNA-shNC (NC) were purchased from GenePharma (Shanghai, China).
3.3. Transient Transfection
Transfections were performed using a Lipofectamine™ 2000 kit (Invitrogen Life Technologies, Carlsbad, CA, USA) following the manufacturer's instructions and our previous study (3). Cells (1 - 3 × 106 per well) grown to a confluency of 50% - 60% in 10-cm Petri dishes were transfected with different double-stranded miRNA mimics, siRNA sequences (600 pmol; GenePharma), or their associated mock sequences. The cells were harvested at 48-h post-transfection.
3.4. Reverse Transcription (RT) Reaction and Quantitative Polymerase Chain Reaction (qPCR)
Total RNAs were purified with the mirVana™ PARIS™ kit (Ambion Life Technologies, Carlsbad, CA, USA). A One-Step SYBR PrimeScript RT-PCR kit (Takara Bio, Inc., Tokyo, Japan) was employed to quantify the copy number of cDNA targets (16).
3.5. BPD Animal Model
All the animals for experiments were approved by the Hospital of Beijing Institutional Animal Care and Use Committee (Beijing, China) and consistent with the guidelines of the National Institutes of Health concerning the care and use of laboratory animals. The experimental BPD mouse model was induced as previously described (17). Newborn Kunming mice (Chinese Academy of Sciences) were placed in sealed Plexiglas chambers and exposed to hyperoxia (60% FiO2) from birth.
3.6. In Vivo Experimental Design
Newborn mouse pups were assigned randomly to one of the four groups: (i) Normoxia (room air, air control group), (ii) hyperoxia plus phosphate-buffered saline (hyperoxia group), (iii) hyperoxia plus BMSCs transfected with negative control (NC) group, and (iv) hyperoxia plus BMSCs transfected with miR-206 (206-group). Each group was composed of six mice. The cells were administered on postnatal day 7 (P7) through an intraperitoneal injection (105 cells/animal) and every following week. The animals were euthanized with intraperitoneal sodium pentobarbital on P35. On P35, the mice were anaesthetized with 50 mg/kg sodium pentobarbital. Blood samples were collected from the orbital sinus, and plasma samples were snap-frozen in liquid nitrogen for later analysis. The lungs were weighed and prepared for histology (17). The lung coefficient (weight of lung/mouse weight) was adopted to compare the degrees of pulmonary edema in each group. Radial alveolar counts (RAC) were also performed as previously described (17).
3.7. Immunofluorescence
Mouse lung tissue samples were incubated with a rabbit polyclonal anti-FN1 antibody (no. 15613-1-AP; Proteintech Group Inc., Chicago, IL, USA) and anti-pulmonary surfactant-associated protein-C (SP-C; SC-13979 lot no. C3111) antibody at a dilution of 1:50 as the primary antibody (16). A goat anti-rabbit immunoglobulin G conjugated with fluorescein isothiocyanate (no.88370, detection for cells; Jackson Immuno Research Laboratories, Inc., West Grove, PA, USA) served as the secondary antibody at a dilution of 1:200. The samples were counterstained with Hoechst 33258 and photographed under a confocal microscope (Nikon C1Si; Nikon, Tokyo, Japan).
3.8. ELISA
Plasma activities of interleukin-6 (IL-6) and TGF-β1 were examined using appropriate ELISA kits (Neobioscience Technology Co.Ltd., Beijing, China).
3.9. Statistical Analysis
Data were presented as the means ± standard deviation of the samples. One-way analysis of variance and the least significant difference test were conducted to compare the differences. P < 0.05 means a statistically significant difference.
4. Results
4.1. Transfection of BMSCs with miR-206 or NC Plasmids In Vitro
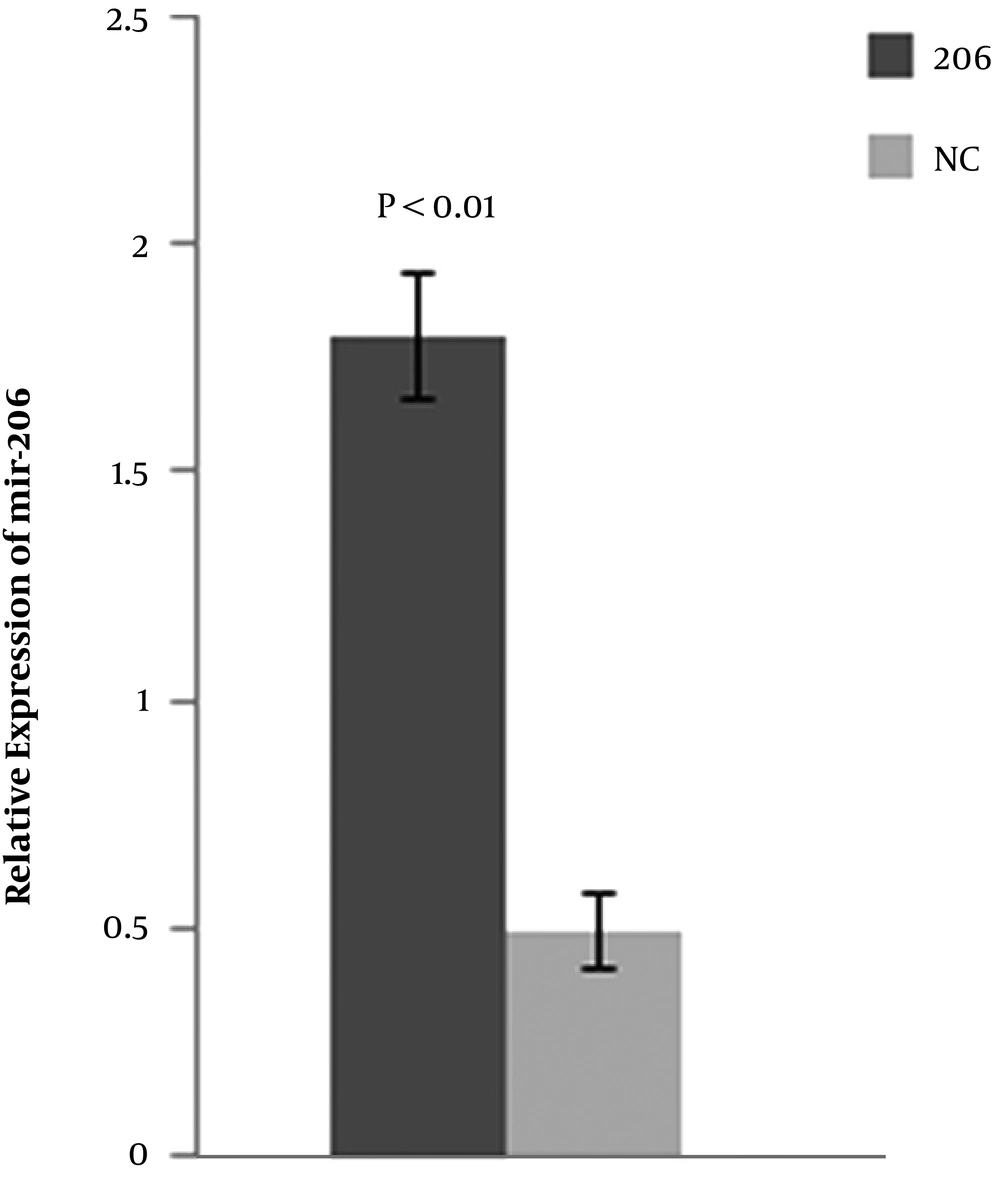
BMSCs were transfected with the miR-206 or NC plasmid. After 48-h post-transfection, the expression level of miR-206 was detected in BMSCs transfected with the miR-206 or NC plasmids by qPCR. The results suggested that compared with BMSCs transfected with the NC plasmid, the BMSCs transfected with miR-206 significantly up-regulated the expression of miR-206 in BMSCs (Figure 1).
4.2. Intraperitoneal Delivery of BMSC Reduces the Degree of Pulmonary Edema and Improves the Alveolarization
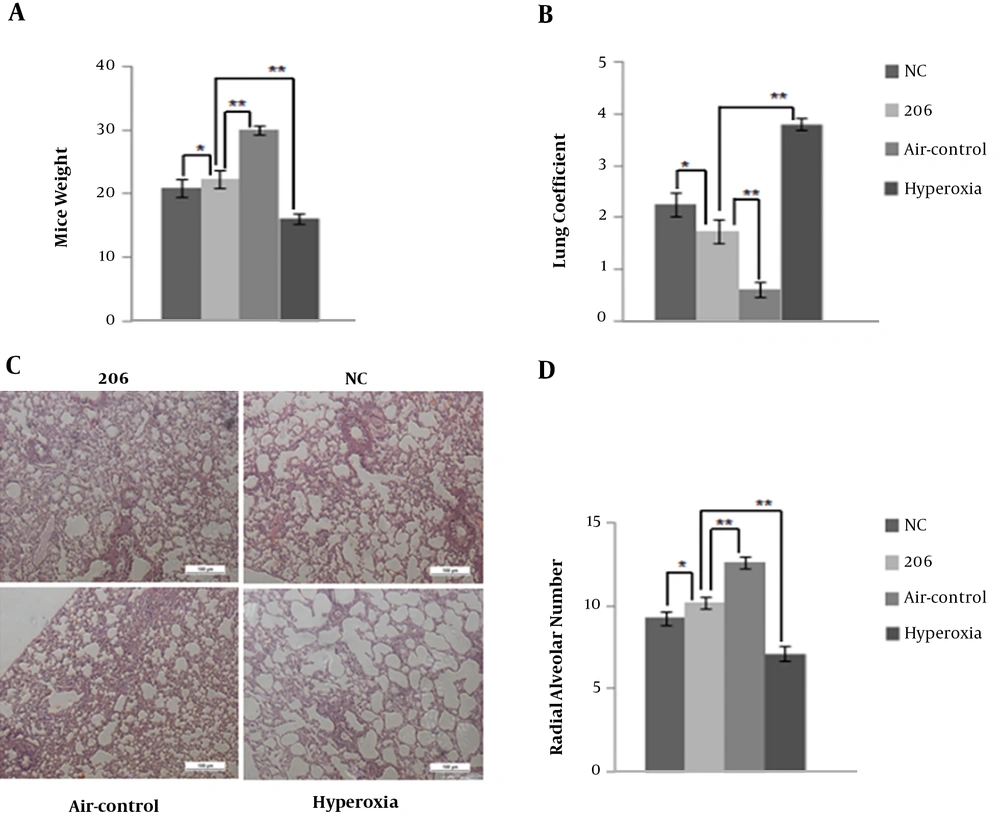
During the experiment, one mouse in the hyperoxia control group died on P32, while the other experimental animals survived. In comparison with the air control group, the BPD mice on P35 had a lower body weight (Figure 2A) and higher lung coefficient (Figure 2B). Figure 2A and B show that BMSCs transfected with the miR-206 or NC plasmid to a certain extent could increase mice weight and decrease the lung coefficient. Mice in the 206-group were heavier with a lower lung coefficient compared with those in the NC group. Through the histological analysis and quantification using RAC, it was observed that intraperitoneal delivery of BMSCs (for BMSCs transfected with miR-206 and with NC plasmid) improved alveolarization compared to the hyperoxia controls (Figure 2C and D). BMSCs transfected with miR-206 were more efficient compared to the BMSCs with the NC plasmid. The comparison between the BMSC-treated group and hyperoxia control group suggests that the BMSCs transfected with the miR-206 or NC plasmid had potential therapeutic benefits in treating BPD.
4.3. Intraperitoneal Delivery of BMSCs Up-Regulates the Expression Levels of SP-C and miR-206
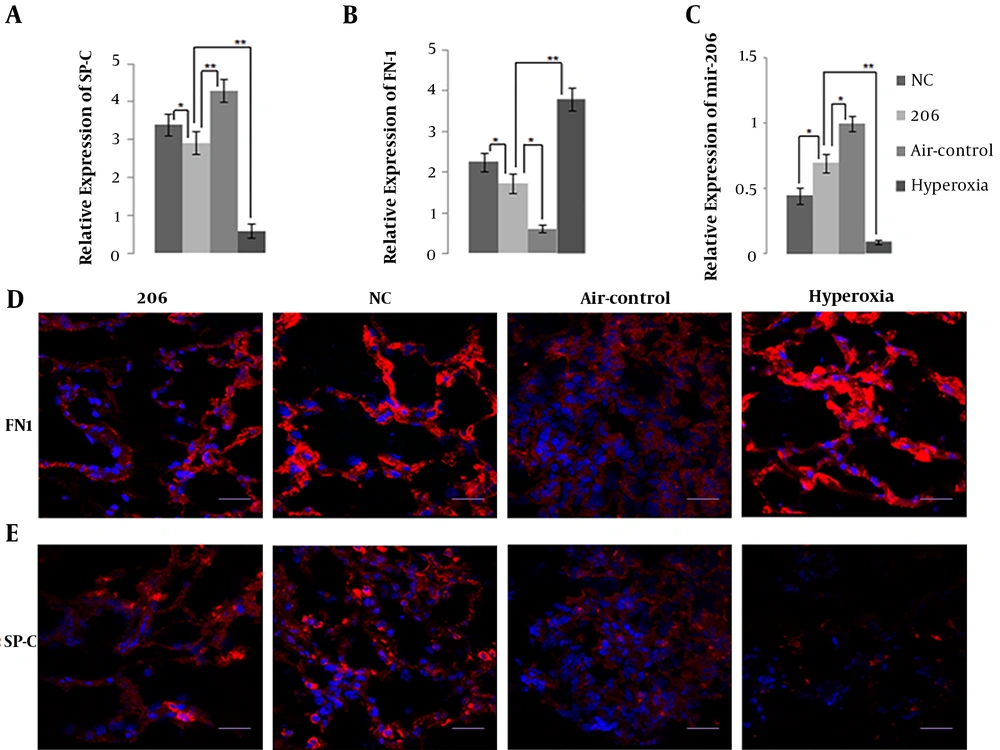
The mRNA expression level by qPCR and protein expression level by immunofluorescence of SP-C, a specific marker of alveolar type II epithelial cell (AEC II) identity that is related to BPD, in BMSC-treated mice lung tissue were significantly higher than in the hyperoxia control group (Figure 3A and E). Obviously, BMSCs transfected with miR-206 down-regulated the expression of SP-C compared to BMSCs with the NC plasmid. Figure 3C shows that intraperitoneal delivery of BMSCs up-regulated the expression level of miR-206 in mice lung tissue, and miR-206 in the 206-group had higher pulmonary expression level than in the NC group, suggesting that BMSCs transfected with miR-206 up-regulated the expression of miR-206.
A, Detection of pulmonary surfactant-associated protein-C (SP-C); B, fibronectin 1 (FN1) and C, microRNA-206 (miR-206) expression in the mice lung tissue of the four groups by quantitative polymerase chain reaction; D, Expression of FN1 and E, SP-C in the mice lung tissue of the four groups by immunohistochemical staining; bars represent 20 μm. * P < 0.05, ** P < 0.01. Abbreviation: NC, negative control.
4.4. In Vivo Intraperitoneal Delivery of BMSC Reduced the Expression Levels of Inflammatory Cytokines
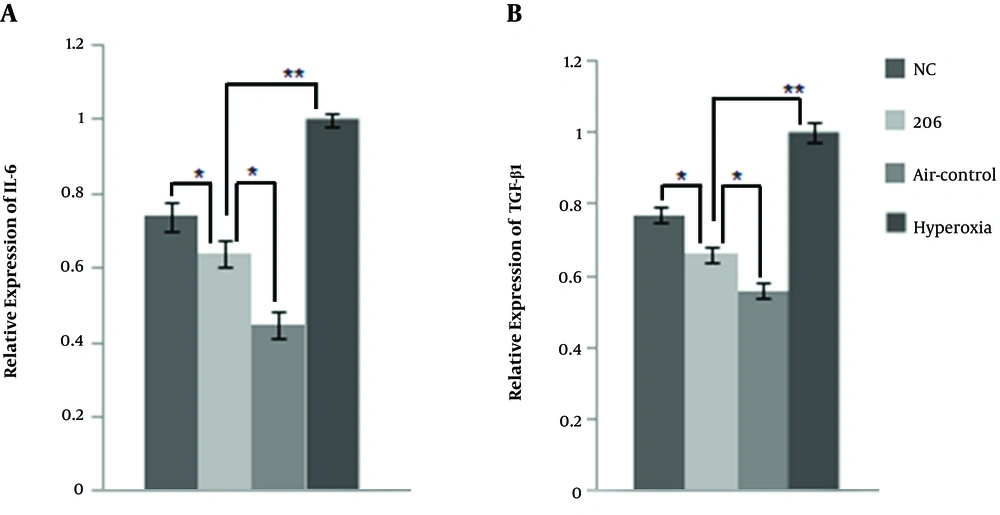
Figure 3B and D show that intraperitoneal delivery of BMSCs down-regulated the expression of FN1 transcriptionally and translationally in BPD mice lung tissue, and BMSCs transfected with miR-206 reduced the expression of FN1 compared to the BMSCs with the NC plasmid. The plasma expression levels of IL-6 and TGF-β1 were lower in the BMSC-treated group than in the hyperoxia control group (Figure 4A and B). In comparison with the BMSCs transfected with the NC plasmid, the BMSCs transfected with 206 down-regulated the plasma expression levels of IL-6 and TGF-β1. It appears that BMSCs may exert their therapeutic benefits by reducing the expression levels of inflammatory cytokines (FN1, IL-6, and TGF-β1).
5. Discussion
This study verified that BMSCs transfected with miR-206 or with the NC plasmid have potential therapeutic benefits in treating BPD by reducing the degrees of pulmonary edema, improving alveolarization, up-regulating the expression level of SP-C, and reducing the gene expression levels of inflammatory cytokines (Fn1, TGF-β1, and IL-6).
miR-206 is related to the diagnosis, treatment, and prognosis of various types of disease, e.g., lung cancer, colorectal cancer, gastric cancer, breast cancer, and osteosarcoma (18-22). The down-regulation of miR-206 and the up-regulation of FN1 observed in patients with BPD and BPD mice are important in ECM remodeling and contribute to the progression of BPD. miR-206 overexpression significantly induced cell apoptosis. It reduced cell proliferation, migration, and adhesion abilities, as well. However, this study found that BMSCs with up-regulation of miR-206 down-regulated the expression of SP-C transcriptionally and translationally compared to the BMSCs with the NC plasmid. This result was also reported by Zhang et al. (23); miR-206 regulates lung surfactant secretion by limiting the availability of vesicle-associated membrane protein-2 (VAMP2) protein. The SP-C is a specific marker of AEC II with a correlation to the development of BPD (24). The down-regulation of SP-C in the lung tissue does not improve the treatment of BPD. Regardless that, BMSCs with up-regulation of miR-206 are more efficient in reducing the degrees of pulmonary edema and pulmonary expression of a number of genes (FN1, TGF-β1, and IL-6) involved in ECM remodeling and fibrosis compared to the BMSCs with the NC plasmid. FN1, TGF-β1, and IL-6 are important for the development of BPD. FN1 is considered to be induced by inflammation (25, 26) and numerous studies have linked the inflammation with an increase in BPD (27, 28). Inhibition of TGF-β1-reduced inflammation and apoptosis may gain insights into the development of potential therapeutic strategies targeted against BPD (29). IL-6 can serve as the independent predictive cytokines of BPD (30). Accordingly, these data support the notion that the inhibition of gene expression levels of inflammatory cytokines (FN1, TGF-β1, and IL-6) improves the treatment of BPD. In general, BMSCs with up-regulation of miR-206 are more beneficial in the treatment of BPD compared to the BMSCs with the NC plasmid. The inhibition of the inflammatory cytokine (Fn1, TGF-β1, and IL-6) gene expression levels may be important for the treatment of BMSCs with the up-regulation of miR-206.
This study also revealed that BMSCs transfected with miR-206 increased the expression level of miR-206 and decreased the expression of FN1 in BPD mouse lung tissue compared to the BMSCs with the NC plasmid, which is more beneficial in treating BPD. This suggested that BMSCs might act as a miR-206 vector in the BMSCs with up-regulation of miR-206 in the treatment of BPD. In addition, compared to the hyperoxia control group, BMSCs transfected with the NC plasmid up-regulated the expression level of miR-206. Whether the therapeutic effect of BMSCs is partially achieved by up-regulating the expression of miR-206 or not needs further investigation. This study provided a novel idea for the treatment of BPD, and miR-206 may become a new target in the treatment of BPD in the future.