Abstract
Introduction:
Triosephosphate isomerase (TPI) deficiency is an autosomal recessive disease and the most severe form of glycolytic enzymopathies, characterized by hemolytic anemia, neurological disorders, infections, and muscle weakness that can affect breathing and heart function. Signs and symptoms include anemia, pallor, jaundice, fatigue, shortness of breath, muscle weakness, atrophy, movement problems, dystonia, tremors, seizures, cardiomyopathy, and diaphragm weakness.Case Presentation:
A three-day female newborn was admitted because of hemolytic anemia. All usual assessments for hemolysis, G6PD, osmotic fragility, RBC morphology and cell panel, minor blood groups, antibody screening, and echocardiography were normal. Neurological manifestations appeared after six months of age, including some seizure-like movements, dystonia, and opisthotonus. Whole exome sequencing confirmed the diagnosis of TPI deficiency with the variant NM-000365.5:c.315G>C chr12-6978338(hg19) which revealed the mutation of p.Glu105Asp. By the age of two years, in addition to some drugs for neurologic manifestations and folic acid, the patient was hospitalized every 30 - 45 days for blood transfusion or because of pneumonia. The episodes of pneumonia increased after 24 months of age, and finally, the child expired at the age of 34 months due to pneumonia and respiratory failure.Conclusions:
Triosephosphate isomerase deficiency should be considered in all neonates with hemolytic anemia without usual causes.Keywords
Triose Phosphate Isomerase Deficiency Anemia Hemolytic Newborn
1. Introduction
Triosephosphate isomerase (TPI) is an enzyme responsible for the metabolism of certain sugars that catalyzes the reversible isomerization and interconversion of glyceraldehyde phosphate and dihydroxyacetone phosphate. This enzyme is essential for glycolysis and the efficient production of energy (1). The related gene is single and located at chromosome 12p13. The enzyme deficiency is inherited in the form of autosomal recessive (2). The TPI deficiency was discovered in the 1960s (3). Although TPI deficiency is rare, it is known as the most severe form of glycolytic enzymopathies, characterized by hemolytic anemia, neurological disorders, infections, and muscle weakness that can affect breathing and heart function. Signs and symptoms include anemia, pallor, jaundice, fatigue, shortness of breath, muscle weakness, atrophy, movement problems such as involuntary muscle contractions (dystonia), tremors, weak muscle tone, seizures, cardiomyopathy, and diaphragm weakness, which may lead to respiratory failure and susceptibility to infections (4-7). The accumulation of dihydroxyacetone phosphate that is chemically converted into toxic methylglyoxal leads to the formation of advanced glycation end products, responsible for clinical signs and symptoms. The TPI deficiency is characterized by decreased enzyme activity And elevated level of dihydroxyacetone phosphate (DHAP). The disease has no effective treatment and frequently leads to death in early childhood (8). Despite passing around 60 years of disease recognition, fewer than 50 cases have been reported (6), and no exact prevalence could be found in the literature.
2. Case Presentation
A three-day female newborn was referred to the Pediatrics Hospital of Qazvin (I.R. Iran) because of anemia. She was a term neonate born to a consanguineous couple through cesarean section delivery. She had a birth weight of 3,360 g, a height of 48 cm, and a head circumference of 35 cm. She was the second offspring in the family, with the first child not having any disease. The laboratory findings on the first and third days after birth are shown in Table 1.
Laboratory Findings on the First and Third Days of Life
| Variables | Values |
|---|---|
| Day 1 | Hgb = 11.5 g/dL, Hct = 36% RBC = 2,800.000/mm3, MCV = 129.4 fL, MCH = 40.8 pg, MCHC = 31.5 g/dL, Platelet = 294,000/mm3, Retic count = 20%, WBC = 60,000 that after correction and subtraction from 36,000 NRBC, the corrected WBC was 24,000/mm3. Bilirubin: Total = 12.2 mg/dL and Direct = 0.77 mg/dL, Blood sugar = 38 mg/dL, Calcium = 8 mg/dL |
| Day 3 | Hgb = 10 g/dL, Hct = 31%, RBC = 2,700.000/mm3, MCV = 127 fL, MCH = 37 pg, MCHC = 29 g/dL, Platelet = 309,000/mm3, Retic count = 22%, Bilirubin: Total = 12.2 mg/dL and Direct = 0.58 mg/dL, Blood Group and Rh: Mother = A+ and Neonate = A+, G6PD level: sufficient, Direct coombs test = Negative, WBC = 14,500 that after correction and subtraction from 1,450 NRBC, the corrected WBC was 13,050/mm3. CRP = 4, Blood culture: No growth, Arterial Blood Gas: Normal. Peripheral Blood Smear: macrocytosis (++), Anisocytosis (++), and Target cells (+) |
The newborn was inserted under phototherapy from the first day of birth at the maternity hospital she was referred from; phototherapy continued at our hospital. Other physical examinations and assessments were performed. After hearing a systolic murmur with the grade II/VI in heart auscultation, echocardiography was done, which revealed a small atrial septal defect. Electrocardiography, chest X-ray, and abdominal sonography showed normal results. In sonography of the pelvis, developmental dislocation of the hip (DDH) was detected on the right side. The result of CBC with a Differential of cells in the newborn’s mother was normal. After three days of assessment at the first stage, the patient was discharged.
The infant was hospitalized once again at the age of 32 days with 4,300 g weight due to jaundice, pallor, restlessness, and poor feeding. Laboratory test results showed WBC = 8000/mm3, Hgb = 5.6 g/dL, RBC = 2,110,000/mm3, MCV = 89 fL, MCH = 26 pg, MCHC = 29.8 g/dL, platelet = 504,000/mm3, retic count = 4%, bilirubin: total = 5.65 mg/dL and direct = 0.34 mg/dL, and negative results in both direct and indirect coombs tests. Glucose 6-phosphate dehydrogenase (G6PD) level was normal. Despite the normal range of MCHC, the osmotic fragility test was done, which was normal. Regarding the above results, the anemia was treated through blood (packed cells) transfusion, and Hgb was increased to 12 mg/dL. Before blood transfusion, blood samples were obtained to check red blood cell (RBC) morphology and cell panel, minor blood groups, and antibody screening. The infant was discharged in good general condition with daily prescribed folic acid. The blood group assessment showed the blood group and Rh of A-Positive with the related antigens of Rh as D+C+c-E-e+(R1R1); minor blood groups were ruled out, and no antibodies were detected. Furthermore, Hb electrophoresis was done, which revealed a normal result.
The third episode of hospitalization was at the age of 7.5 months when the infant, in addition to pallor, cough, nausea, and vomiting, had some seizure-like movements; dystonia, and opisthotonus. Based on the parents' declaration, the neurologic involvement began after the age of six months. At admission, the patient had an 8.5 kg weight (below +1 SD curve), 71 cm height (above +1 SD curve), and head circumference of 45.5 cm (0n +2 SD curve). The CBC showed WBC = 14,900/mm3, RBC = 2,900,000/mm3, Hgb = 7 mg/dL, Hct = 21%, MCV = 92 fL, MCH = 24 pg, MCHC = 26 g/dL, NRBC = 8%, and Plat. = 479,000/mm3. The levels of lactate and ammonia in venous blood were 21.5 mg/dL and 0.5 micromol/L, respectively, which were in the normal range. Brain MRI revealed brain atrophy and delayed myelination, but Electroencephalography (EEG) was normal. The infant was discharged with the medical prescription of 1 mg of Artane (Trihexyphenidyl) daily.
The laboratory result of whole-exome sequencing was delivered when the patient was eight months old, which confirmed the diagnosis of triosephosphate isomerase deficiency. The disease variant was reported as NM-000365.5:c.315G>C chr12-6978338(hg19), which reveals the mutation of p.Glu105Asp.
The infant was admitted to the hospital due to severe pneumonia at the age of 9 months, and it was the only hospitalization for a different reason. The next hospitalization was at the age of 10 months because of pallor and restlessness. The infant was not able to sit or roll. Audiometry (ABR) was done, which showed normal hearing. Laboratory assessment showed Hgb = 7.2 mg/dL, Hct = 21.7%, RBC = 2,870,000/mm3, MCV = 94 fL, MCH = 25 pg, MCHC = 26.6 g/dL, Plat. = 437,000/mm3, and LDH = 3,300 U/L. The patient was discharged after receiving packed cells and administration of clobazam 2.5 mg/q8h and daily folic acid. Hemoglobin electrophoresis that was checked around one year old showed a normal result.
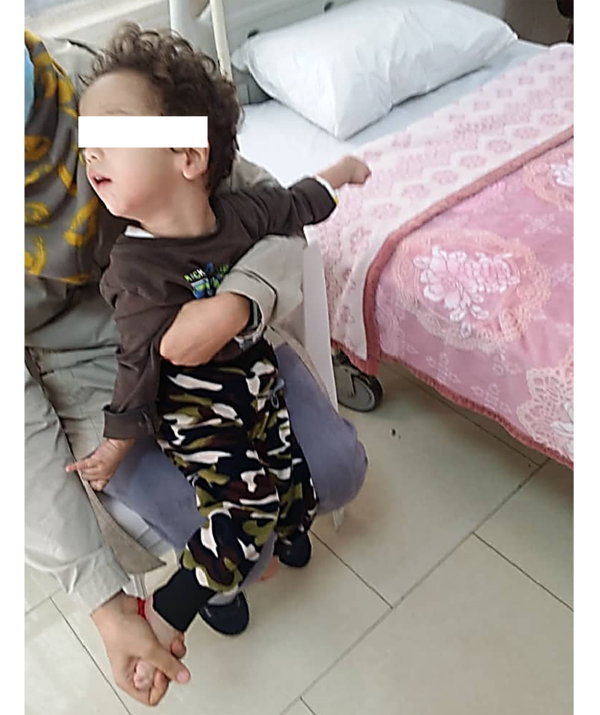
By the age of two years old (Figure 1), the patient was admitted to the hospital every 30 - 45 days for blood transfusion; during this time, some episodes of pneumonia occurred. The number of respiratory infections increased after 24 months of age, and finally, the child expired at the age of 34 months due to pneumonia and respiratory failure.
The reported patient with TPI deficiency at the age of two years old. The picture shows dystonia in the upper limbs and cervical dystonia. Lower limbs were affected mildly.
3. Discussion
Although the disease is rare, some TPI deficiency mutations are reported globally (such as Val231Met and Arg189Gln mutations). The Glu104Asp mutation (9, 10) is the most frequent one, which is nearly the same as our case. Since the genetic pattern of TPI deficiency is autosomal recessive, our patient has indeed been homozygous. Concerning the lack of any previous report of this enzymopathy, it seems to be the first reported case from the I.R. Iran. In some accounts, recurrent lower respiratory infections are a significant problem before progressive neurological involvement (11). This was not true in our case, who had neurologic manifestations before the episodes of pneumonia, for which we could not find out a reason. In addition to the signs and symptoms that mentioned before, intellectual disability is reported in these patients (9).
Chorionic villus DNA analysis makes prenatal diagnosis in the first trimester of pregnancy possible. This has been done in a Spanish family with Glu104Asp mutation TPI deficiency, in which the fetus was heterozygous for the mutation and therefore predicted to be clinically unaffected (12).
Although some studies emphasize the absence of any treatment for the disease (10), there is some report about successful treatment of the disease in mice with similar human TPI deficiency through bone marrow transplantation (13). Although the disease is rare and is not routinely screened because of the low probability of occurrence, this enzymopathy should be considered in each newborn with the presentation of coombs-negative hemolytic anemia without recognizing any particular reason.
Comparing laboratory data on the first and third days of life reveals prominent anemia on the first day and a rapidly progressive decrease in Hgb and Hct levels accompanied by an increasing percentage of reticulocyte count.
As mentioned above, some advantages are obtained in treating the disease in animal models through bone marrow transplantation. In the condition of inevitable mortality in early childhood, it looks pretty logical that this treatment is considered for the patients before appearing neurologic manifestations, emphasizing the early diagnosis of the disease.
We could not assess the parents for genetic analyses, partly because of the family's economic limitations. It may be considered the only limitation of the present study.
References
-
1.
Orosz F, Olah J, Ovadi J. Triosephosphate isomerase deficiency: facts and doubts. IUBMB Life. 2006;58(12):703-15. [PubMed ID: 17424909]. https://doi.org/10.1080/15216540601115960.
-
2.
Glader B. Other Hereditary Red Blood Cell Disorders. In: Rimoin D, Pyeritz R, Korf B, editors. Emery and Rimoin's Principles and Practice of Medical Genetics. 6th ed. Massachusetts, USA: Academic Press; 2013. p. 1-25. https://doi.org/10.1016/b978-0-12-383834-6.00076-8.
-
3.
Segal J, Mulleder M, Kruger A, Adler T, Scholze-Wittler M, Becker L, et al. Low catalytic activity is insufficient to induce disease pathology in triosephosphate isomerase deficiency. J Inherit Metab Dis. 2019;42(5):839-49. [PubMed ID: 31111503]. [PubMed Central ID: PMC7887927]. https://doi.org/10.1002/jimd.12105.
-
4.
MedlinePlus. Triosephosphate isomerase deficiency. Maryland, USA: National Library of Medicine; 2014. Available from: https://medlineplus.gov/genetics/condition/triosephosphate-isomerase-deficiency/.
-
5.
Orphanet. Triose phosphate-isomerase deficiency. Paris, France: Orphanet; 2012. Available from: https://www.orpha.net/consor/cgi-bin/OC_Exp.php?lng=en&Expert=868.
-
6.
Harris C, Nelson B, Farber D, Bickel S, Huxol H, Asamoah A, et al. Child Neurology: Triosephosphate isomerase deficiency. Neurology. 2020;95(24):e3448-51. [PubMed ID: 32873690]. https://doi.org/10.1212/WNL.0000000000010745.
-
7.
Roland BP, Zeccola AM, Larsen SB, Amrich CG, Talsma AD, Stuchul KA, et al. Structural and Genetic Studies Demonstrate Neurologic Dysfunction in Triosephosphate Isomerase Deficiency Is Associated with Impaired Synaptic Vesicle Dynamics. PLoS Genet. 2016;12(3). e1005941. [PubMed ID: 27031109]. [PubMed Central ID: PMC4816394]. https://doi.org/10.1371/journal.pgen.1005941.
-
8.
Orosz F, Olah J, Ovadi J. Triosephosphate isomerase deficiency: new insights into an enigmatic disease. Biochim Biophys Acta. 2009;1792(12):1168-74. [PubMed ID: 19786097]. https://doi.org/10.1016/j.bbadis.2009.09.012.
-
9.
Serdaroglu G, Aydinok Y, Yilmaz S, Manco L, Ozer E. Triosephosphate isomerase deficiency: a patient with Val231Met mutation. Pediatr Neurol. 2011;44(2):139-42. [PubMed ID: 21215915]. https://doi.org/10.1016/j.pediatrneurol.2010.08.016.
-
10.
Roland BP, Richards KR, Hrizo SL, Eicher S, Barile ZJ, Chang TC, et al. Missense variant in TPI1 (Arg189Gln) causes neurologic deficits through structural changes in the triosephosphate isomerase catalytic site and reduced enzyme levels in vivo. Biochim Biophys Acta Mol Basis Dis. 2019;1865(9):2257-66. [PubMed ID: 31075491]. [PubMed Central ID: PMC6659405]. https://doi.org/10.1016/j.bbadis.2019.05.002.
-
11.
Aissa K, Kamoun F, Sfaihi L, Ghedira ES, Aloulou H, Kamoun T, et al. Hemolytic anemia and progressive neurologic impairment: think about triosephosphate isomerase deficiency. Fetal Pediatr Pathol. 2014;33(4):234-8. [PubMed ID: 24840153]. https://doi.org/10.3109/15513815.2014.915365.
-
12.
Repiso A, Corrons JL, Vulliamy T, Killeen N, Layton M, Carreras J, et al. New haplotype for the Glu104Asp mutation in triose-phosphate isomerase deficiency and prenatal diagnosis in a Spanish family. J Inherit Metab Dis. 2005;28(5):807-9. [PubMed ID: 16151918]. https://doi.org/10.1007/s10545-005-0098-6.
-
13.
Conway AJ, Brown FC, Hortle EJ, Burgio G, Foote SJ, Morton CJ, et al. Bone marrow transplantation corrects haemolytic anaemia in a novel ENU mutagenesis mouse model of TPI deficiency. Dis Model Mech. 2018;11(5). [PubMed ID: 29720471]. [PubMed Central ID: PMC5992613]. https://doi.org/10.1242/dmm.034678.