1. Introduction
Mucormycosis is rare in urinary tract infections. Renal mucormycosis is considered in immunocompromised patients. Most cases of mucormycosis involve tissue invasion (1, 2). In the present case, renal mucormycosis was confined to the left unilateral kidney, and his right kidney was intact without fungus balls or atrophy for ten years. Patients with renal mucormycosis typically present with fever, flank pain, hematuria, urinary obstruction, and negative urine culture results. Bilateral disease is usually fatal. Unlike the typical presentation of renal mucormycosis, the patient in our study did not have any fever, urgency, frequency, dysuria or anuria, suprapubic discomfort, flank pain, or growth retardation.
2. Case Presentation
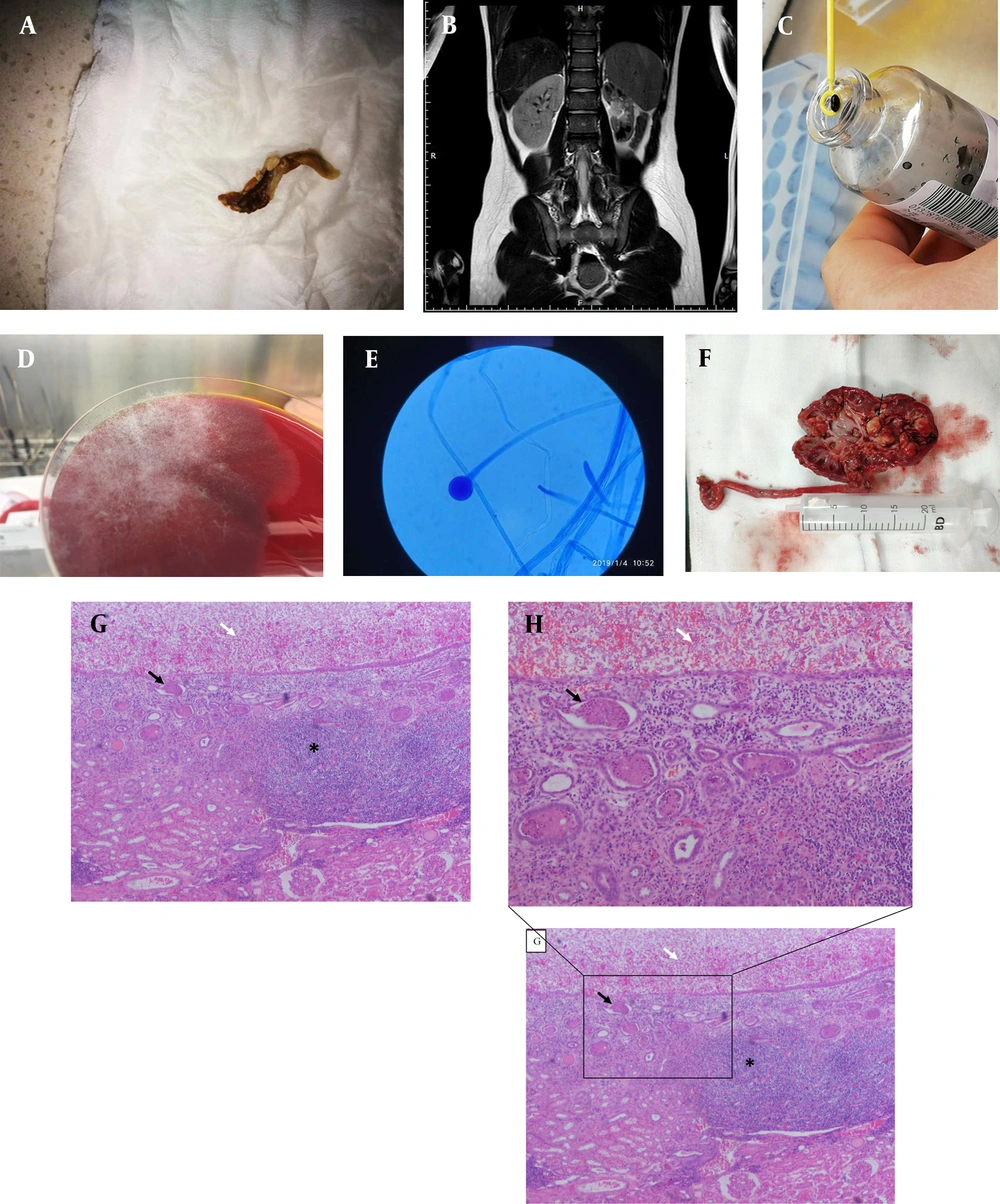
An 11-year-old boy was admitted to the Xinhua Hospital affiliated with Shanghai Jiaotong University School of Medicine with macroscopic pyuria for ten years. He was a premature (gestational 35+2 weeks) baby. Two days after birth, he was admitted to a local hospital for neonatal jaundice. On day 7, after hospitalization, fever and pyuria were observed. However, midstream urine cultures were negative, even after several detections. Ultrasonography showed an enlarged left kidney, parenchymal echo thickening and enhancement, and hydronephrosis. Broad-spectrum antibiotics included cefoperazone, cefoperazone-sulbactam, and vancomycin; the fever disappeared while pyuria persisted. He was admitted to different medical centers several times for pyuria, albeit without improvement. The patient had no fever, urgency, frequency, dysuria, suprapubic discomfort, or flank pain except pyuria. Cystography was performed when he was 2.5 years old, and the result was negative for vesicoureteral reflux (VUR). The patient was diagnosed as having asymptomatic bacteriuria. He was advised to undergo follow-up urinalysis and ultrasound every year without any treatment. During this follow-up, macroscopic pyuria and transient dysuria were detected. A dense, tan-colored, rubbery mass (measuring 2 × 0.3 cm2) was found in the uria within the year (Figure 1A). Moreover, ultrasound showed light hydronephrosis in the left kidney, which was smaller than that in the right kidney. The pelvis had an increased echotexture and loss of corticomedullary differentiation, while the right kidney was nearly normal. When he was ten years old, the Tc-99m-mercaptoacetyltriglycine (MAG3) scan confirmed a mute left kidney and satisfactory compensatory function on the right (left estimated glomerular filtration rate [eGFR] 8.2 mL/min, right eGFR 58.35 mL/min). The patient was admitted to our hospital for further treatment. The patient was not in distress, and his nasal mucosa and oropharynx appeared normal. The results of cardiac, pulmonary, and abdominal examinations were unremarkable. Urinalysis revealed a high number of erythrocytes (164/high-power field (HPF); normal range ≤ 3/HPF) and leukocytes (50 - 60/HPF; normal range 0 - 5/HPF). Leukocyte esterase level was high, whereas urine nitrite level was negative. Creatinine level was 69 μmol/L (normal range < 97 μmol/L), and the estimated GFR was 58 mL/min/1.73 m2 (normal range > 90 mL/min/1.73 m2). Magnetic resonance urography (MRU) showed left renal atrophy and hydronephrosis with suspicious calculus in both kidneys’ left collecting system, upper ureter, and renal cyst formation (Figure 1B).
A, Dense, tan-colored, and rubbery mass (measuring 2 × 0.3 cm) found in the uria. B, Suspicious calculus in the collecting system and in the upper ureter of the left kidney by magnetic resonance urography (white arrow). C, Pathogen proliferated in the blood culture bottle for 24 h. D, Rhizopus oryzae proliferated on blood agar at 35 °C for 48 h with a white cottony morphology. E, Lactic acid phenol cotton blue staining tease mount preparation of the fungal culture showing aseptate hyphae, rhizoids, and sporangia/sporangiophore morphology, classically found in the members of the Rhizopus genus (100×). F, Dissected segments of the kidney revealing fungal masses in the renal pelvis and calyces (black arrow). G, Pathology of nephrectomy showing many hyphae of fungus balls caused by Rhizopus in the left pelvicalyceal dilation (white arrow) and infiltration of a large number of lymphocytes in the submucosa (asterisk *) and cellular casts in the medullary renal tubules (black arrow) (40×). H, Pathology of nephrectomy showing many hyphae of fungus balls caused by Rhizopus in the left pelvicalyceal dilation (white arrow) and cellular casts in the medullary renal tubules (black arrow) (Zoom in from part of picture G, 100×)
The patient received a wide range of intravenous injections or oral antimicrobial agents for presumptive bacterial pyelonephritis in the first 15 days after admission, including ceftriaxone (days 1 - 5), cefepime (days 5 - 10), meropenem, and fosfomycin (days 10 - 14). However, the patient’s clinical status did not improve. Second, he received fluconazole prior to definitive laboratory identification of the pathogens. Several clean middle-stream urine cultures were negative. Twenty mL of fresh urine specimens obtained from suprapubic bladder puncture instead of a clean middle stream were cultivated in a blood culture bottle to increase the positive urine culture rate of the pathogens. Second-generation sequencing of bacterial DNA from fresh urine specimens was simultaneously performed. The pathogens grew rapidly in the blood culture bottles (Figure 1C). Rhizopus oryzae grew rapidly on blood agar at 35°C for 48 h with a white, cottony morphology (Figure 1D). Lactic acid phenol cotton blue staining tease mount preparation from the fungal culture revealed aseptate hyphae, rhizoids, and sporangia/sporangiophore morphology, classically found in the members of the genus Rhizopus (100× magnification) (Figure 1E). Subsequent fungal pyelonephritis led to a change in treatment with amphotericin B. After one week of treatment, a clean middle stream urine culture revealed Stenotrophomonas maltophilia and Chryseobacterium indologenes. These two bacteria were sensitive to sulfamethoxazole/trimethoprim (SMZco). The clinical signs and symptoms of the patient improved transiently over the week following treatment with amphotericin B and oral SMZco. The patient exhibited a whole-body rash and fever after oral SMZco. Renal function evaluation revealed a gradual increase in creatinine levels from 69 to 97 μmol/L. Subsequently, SMZco administration was discontinued because of allergy, and the dose of amphotericin B was reduced to half; also, pyuria persisted. Additionally, urine culture was positive for S. maltophilia and C. indologenes. The patient underwent nephrectomy because urine amphotericin B concentrations were too low to be effective. Subsequently, pyuria disappeared immediately without additional bladder irrigation or amphotericin B treatment, and urinalysis was normal during follow-up. Pathology of specimens from nephrectomy showed a large number of fungus balls caused by Rhizopus in the left pelvicalyceal dilation, infiltration of a large number of lymphocytes in the submucosa, and cellular casts in the medullary renal tubules (Figure 1F and 1G). The hyphae were found in specimens from nephrectomy (Figure 1H). Written informed consent was obtained from the patient’s family members.
3. Discussion
This is the first reported case of an invasive fungus ball caused by Rhizopus confined to the kidney of a boy without congenital urinary tract malformations for approximately ten years. Mucormycosis primarily manifests as cutaneous, gastrointestinal, rhinocerebral, and pulmonary infections in children (3). Although urinary candidiasis is one of the most common fungal infections of the urinary tract, mucormycosis can also be a rare urinary tract infection (4). Renal mucormycosis is considered in immunocompromised patients. The most common risk factors for mucormycosis are older age, female sex, broad-spectrum antibiotic use, uncontrolled diabetes, diabetic ketoacidosis, long-term steroid therapy, hematological malignancy, defective host innate or cell-mediated immunity, urinary drainage devices, prior surgical procedures, and diabetes mellitus (5, 6). Patients with uncontrolled diabetes and iron overload are susceptible to mucormycosis (7). The patient in the current study was a healthy child without a history of diabetes or iron overload. Additionally, cystography results were negative for VUR. Patients with renal mucormycosis typically present with fever, flank pain, hematuria, urinary obstruction, and negative urine culture results. The patient had no fever, urgency, frequency, dysuria or anuria, suprapubic discomfort, flank pain, or growth retardation. These features differ from those of other mucormycosis (3, 6).
Identifying Rhizopus species isolated from urine culture is a crucial step for treatment. Conventional clean middle-stream urine culture was performed several times without any positive results. Using a blood culture bottle for urine culture could increase the positivity rate. In this case, we collected 20 mL of fresh urine specimens obtained from suprapubic bladder puncture and injected them into a blood culture bottle for urine culture. Consequently, we detected pathogenic bacteria using next-generation sequencing. Urine culture in blood culture bottles and next-generation sequencing revealed R. oryzae (8), which is the most common fungal species causing mucormycosis (9, 10). A renal fungus ball is a mixture of fungal and sloughed renal epithelial cells that form a mass obstructing the urinary tract. Fungus balls are common in tract infections. A dense, tan-colored, rubbery mass (measuring 2 × 0.3 cm2) was found in the uria almost every year (Figure 1A). Fungus balls were observed in the left kidney after nephrectomy (Figure 1F). The mass and fluid were subjected to bacterial and fungal culture, respectively. The fungus ball was bisected longitudinally and dissected into several segments for microscopic analysis. The fungal culture grew rapidly in blood culture bottles (Figure 1C). A lactophenol cotton blue-stained tease preparation (Figure 1D) from the fungal culture revealed aseptate hyphae, rhizoids, and sporangia/sporangiophore morphology, classically found in the members of the genus Rhizopus (100× magnification). Identifying a Rhizopus species fungus ball in the kidney (Figure 1G) and the subsequent fungal pyelonephritis led to a change in treatment with amphotericin B (10).
In the present case, Rhizopus species were isolated and identified from urine culture. Fluconazole and amphotericin B are practical options for R. oryzae in renal tract infections. Other antifungal agents, such as voriconazole, posaconazole, itraconazole, and echinocandins, are not recommended for lower urinary tract infections because few active drugs are excreted in the urine. Although fluconazole is excreted 80% unchanged in the urine in its active form, R. oryzae is resistant to fluconazole. Amphotericin B exhibits fungicidal activity and adequate penetration into biofilms on vascular devices (11, 12). The patient was admitted with amphotericin B treatment for approximately one and a half months. Although urinalysis showed complete recovery for a short duration during systemic amphotericin B therapy, many fungus balls were found during nephrectomy (Figure 1F). The amount of active amphotericin B excreted in the urine is lower because of the low GFR.
Bilateral disease is usually fatal. In the present case, mucormycosis was an isolated renal infection. The patient’s right kidney was intact without fungus balls or atrophy for ten years. In addition, we carried out next-generation sequencing of the blood to exclude systemic infections (data not shown). Nephrectomy and systemic amphotericin B therapy are common treatment regimens for localized unilateral disease (13). Nephrotoxicity due to the administration of amphotericin B was observed during the treatment. The creatinine level was elevated within five days of starting the treatment but recovered by omitting doses. The nephrotoxicity of amphotericin B was reversible. Although percutaneous drainage and systemic antifungal therapy may clear the fungus ball, some patients require additional interventions. Renal pelvic irrigation with antifungal agents might clear the remaining debris; however, irrigation can cause increased intrarenal pressure and sepsis, making irrigation contraindicated in patients with distal obstruction. Finally, the patient underwent nephrectomy, and the pyuria disappeared without bladder irrigation and additional amphotericin B treatment. The urinalysis results were normal during follow-up.
This report presented the first case of a non-invasive fungus ball caused by a Rhizopus species confined to the unilateral kidney in a healthy boy for approximately ten years. Therefore, clinicians need to have a high index of suspicion for fungal infections in refractory urinary tract infections. Next-generation DNA sequencing technology can be used for an accurate diagnosis.