1. Introduction
Double Aortic Arch (DAA) is a rare congenital cardiovascular abnormality. The incidence of this condition in the general population is common at 0.1%. Double Aortic Arch is caused by the failure of involution of the right aortic branch that remains beyond the embryonic stage. These two separate aortic arches may be connected, causing a vascular ring to form (1, 2). This vascular ring compresses the trachea and esophagus and causes respiratory symptoms such as stridor, wheezing, coughing, or choking. Some Double Aortic Arch patients present with gastrointestinal and cardiac symptoms such as regurgitation, dysphagia, murmur, cyanosis, or chest pain (3).
Imaging is the method of choice to confirm the diagnosis of a double aortic arch. This includes trans-thoracic echocardiography, cardiac MRI (magnetic resonance imaging), and CT (computed tomography) angiography (4).
In the present report, the case of a 10-year-old boy was evaluated for acute respiratory symptoms, abnormal examinations, and failure to respond to outpatient treatment.
2. Case Presentation
A 10-year-old boy was admitted to our hospital’s pediatric center with a chief complaint of recurrent cough and fever. The patient had a history of recurrent respiratory infection, and symptoms relieved by routine treatment. On admission, the patient also complained of shortness of breath, general fatigue, and chest pain; his symptoms worsened at night. There was no history of foreign body aspiration, weight loss, and trauma. Also, there was no history of a congenital anomaly or respiratory dysfunction in a sibling.
Physical examination determined his weight as 25 kg, which was underweight for his age. The saturation level of oxygen was 96% without supplemental oxygen therapy; and tachypnea, suprasternal retraction, chest wall deformity, as well as respiratory and expiratory stridor were detected in upper airways, which can be only noticed through auscultation, crackle in lungs, and heart sounds (S1 and S2) providing that they have the regular pattern, and both are auscultated more clearly at the right side with a grade I/VI murmur. Since the symptoms of the infection were reduced and improved by administering the outpatient treatment, the patient was not fully evaluated for the cause of recurrence of the respiratory infection. However, the patient was hospitalized due to the persistence of symptoms and non-response to outpatient treatment, as well as the abnormal examination findings.
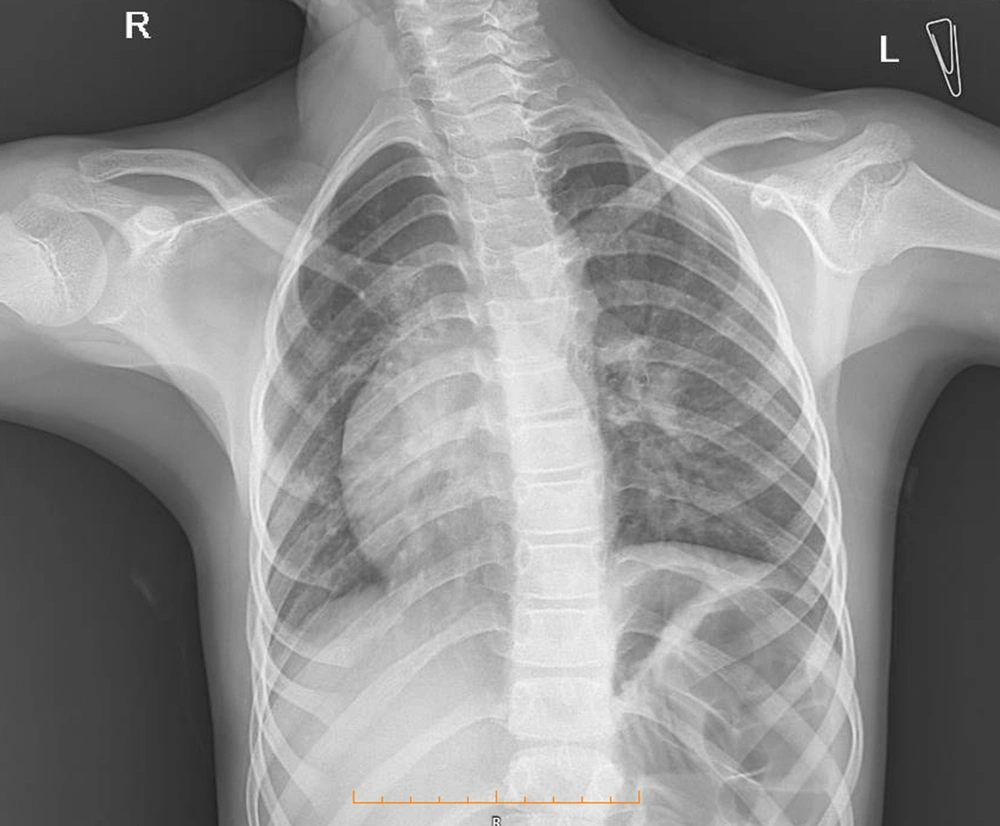
Due to the recurrent respiratory infection in the patient, therefore, chest radiography was performed, which showed dextrocardia but did not confirm the abnormality in the lung field (Figure 1). Due to dextrocardia and murmur sound in cardiology physical exam, consultation with a pediatric cardiac specialist was recommended. According to pediatric cardiologist consultation, ECG and echocardiography were requested. A cardiac evaluation showed no pathologic findings. An electrocardiogram (ECG) revealed normal sinus rhythm and right axis deviation. Echocardiography only confirmed dextrocardia along with normal pulmonary veins, normal size four chambers, septa with normal ejection fraction (EF); however, no valvar dysfunction was observed.
Abdominal and kidney sonography was normal, with no signs of situs inversus totalis. Due to the patient's chief complaint and the fact that tuberculosis was expected in this area, a purified protein derivative test (PPD) was performed to examine tuberculosis, which proved negative. The patient we also examined for immunocompromised diseases. According to the results from laboratory tests, the leukocytosis, Anemia, and Erythrocyte sedimentation rates (ESR) was 40, and the serum levels of IgE, IgA, IgM, and IgG were normal; the spiral CT scan of Paranasal sinuses showed retention cyst and infected concha bullosa, which was unspecified and negative for immunocompromised diseases. Because of the stridor sound in the physical exam, a pulmonary function test (PFT) was also recommended; the result suggested a variable intrathoracic upper airway obstruction.
Then bronchoscopy was performed on the patient, which confirmed tracheomalacia in the upper zone of the trachea; and the bronchoalveolar lavage (BAL) specimen was taken for evaluation. According to the BAL result, the Adenosine deaminase level was normal, while smear and culture were negative for tuberculosis and other bacterial infections.
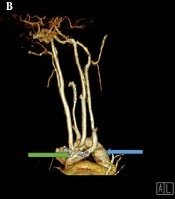
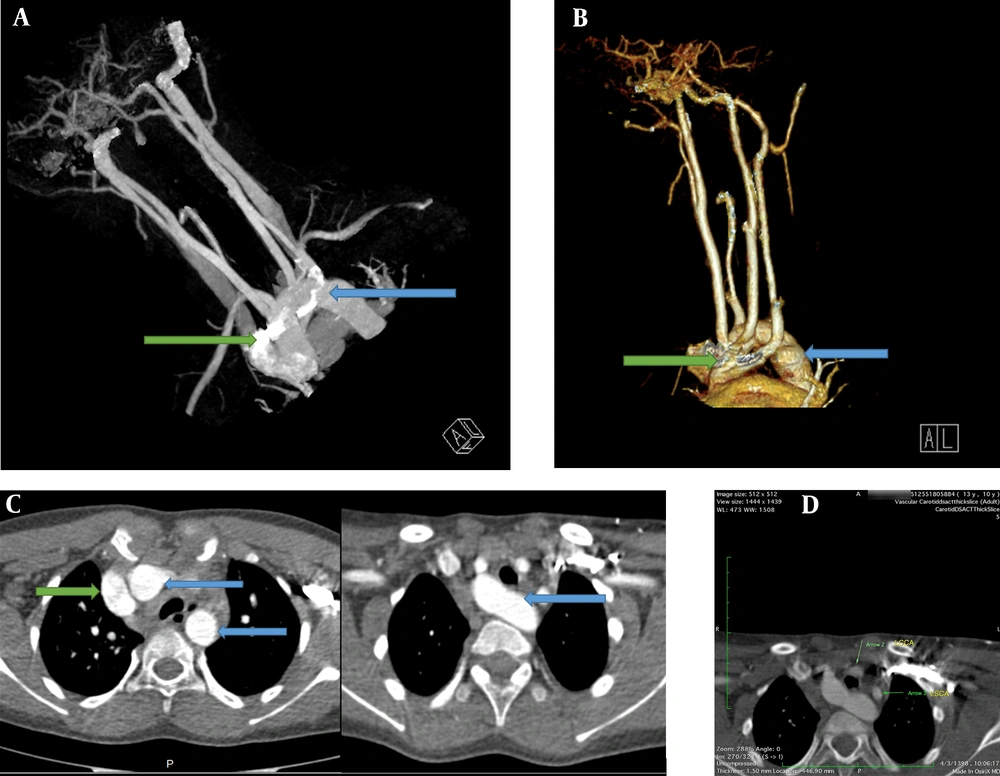
Due to the tracheomalacia, stridor sound in the physical exam, and the PFT pattern, the patient was recommended to have an enhanced computed angiography in order to collect more accurate information and confirm the diagnosis of double aortic arch (Figure 2A-C). As for the neck and mediastinum, CT angiography revealed that the aortic arch had passed through the posterior of the trachea and esophagus. Four different branches originate from the aortic arch, which include the left subclavian branch, the left and right common carotid branches, and the right subclavian branch. The left subclavian artery and the left common carotid artery had passed through the anterior of the trachea and were close to the posterior end of the aortic arch; furthermore, a slight prominence was seen in the adjacent region mentioned in the posterior aortic arch. These findings were suggestive of double aortic with arteritic segment (Figure 2D).
A, Chest computed tomography with IV contrast. An anterior view; shows a double aortic arch (blue arrow and green arrow). B, Computerized tomography reconstruction arteriography, anterolateral view; shows both double aortic arch (green and blue arrows). C, Chest computed tomography with IV contrast showed a double aortic arch passes through the posterior of the trachea and esophagus (blue arrows original aortic arch, green arrow shows the second arch). D, Mediastinal view of chest computed tomography showed that the left subclavian artery and the left common carotid artery pass through the anterior of the trachea and are close to the posterior end of the aortic arch. LCCA, left common carotid artery; LSCA, the left subclavian artery.
The anterior and posterior arches of the aorta had a compressive effect on the trachea and esophagus and the heart axis, apparently, was tilted to the right. However, the heart cavities were not imaged and, therefore, it was not possible to accurately evaluate the relationship between the heart cavities and the apex location. During the admission, the patient was treated for pneumonia and he was recommended, due to his stable condition, to have elective cardiovascular surgery after discharge from the hospital.
3. Discussion
A double aortic arch (DAA) is a common vascular complete ring miscreation type. It occurs due to the failure of the fourth embryonic branchial arch to drift back. In this condition, the ascending aorta is separated into a left and right arch, in which they merge to encircle the trachea and esophagus fully (5, 6).
It is important to recognize all kinds of aortic arch abnormalities because they may be associated with abnormal conditions, like vascular rings, congenital heart disease, and chromosomal mutations. These related conditions also can affect prognosis, management, and all kinds of therapeutic interventions in patients (7).
Paying enough attention to a newborn with respiratory symptoms is the key to diagnosing vascular ring compression accurately, especially when the patient has signs and symptoms of laryngomalacia. However, no abnormality was observed in our patient during our bronchoscopic investigation. The vascular compression of the trachea and esophagus can lead to respiratory and gastrointestinal signs and symptoms, which can vary from stridor, persistent cough, wheezing, and recurrent respiratory infection to feeding difficulties, nausea, and vomiting, mainly in the period of infancy (3, 8).
Most cases of DAA display symptoms during infancy, and it is hard to find an adult patient with DAA symptoms, peculiarly among the elderly. Majority of adults diagnosed with DAA have a history of exertional dyspnea and dysphagia; however, they used to be wrongly diagnosed with conditions like asthma, chronic obstructive pulmonary disease (COPD), or intrinsic cardiac disease in the past (9, 10).
In case when a patient is suspected to be afflicted with DAA, valuable diagnostic modalities like echocardiography, cardiac magnetic resonance (MR) imaging, and computed tomographic (CT) angiography should be used in order to confirm aortic arch abnormalities and malformations. Today, these modalities are preferred to catheter angiography and barium swallow due to their being noninvasive (7).
The patient in our case had a fever, recurrent cough, shortness of breath, paleness, tachypnea, respiratory stridor, and expiratory crackles in the lungs. His symptoms started at the age of two, but improved by routine treatment such as Bronchodilator, systemic corticosteroids, and Antibiotics. Therefore, he had been misdiagnosed for a long time due to the fact that early diagnosis of DAA is difficult and rare. Generally, DAA is suspected after ruling out the causes of airway obstruction and respiratory disease.
In most cases, a double aortic arch is diagnosed during pregnancy or infancy, and the practitioners rarely fail to diagnose it in childhood. The presence of a cardiac anomaly with a double aortic arch is rare; however, the patient in our study had dextrocardia. In studies with double aortic arch diagnosis, most patients suffered from acute respiratory symptoms and stridor; however, the patient in our study had respiratory symptoms and his weight percentile was lower than the normal level due to the disease, which had not been reported in other studies.
Our study faced few limitations. First, the patient was transferred to advanced centers for imaging and implementing necessary procedures due to the lack of advanced diagnostic tools in the initial treatment center. Second, the patient was an immigrant to this country, and, therefore, no proper follow-up plan was executed; in other words, the patient returned to his country soon after his discharge, and it was impossible to follow the patient's surgery, unfortunately.
Taking into account the fact that any delay in the diagnosis of double aortic arch increases the mortality and advanced complications in infants and children, it was recommended, in our study, that this diagnosis should be added to the differential diagnosis for dealing with unexplained stridor and recurrent respiratory infection.