1. Background
Congenital heart disease (CHD) is the most common developmental anomaly (4 – 10 per 1,000 live births), representing an important noninfectious cause of death in children. Patients with CHD are at higher risk of infections (1). Many of them will undergo cardiac surgery, whose outcomes depend on many preoperative, intraoperative, and postoperative factors. Preoperative factors include age, sex, type of cardiac anomaly, concomitant non-cardiac disorders (pulmonary hypertension, renal dysfunction), and transfusion of blood products. Intraoperative factors involve intraoperative complications, cardiopulmonary bypass (CPB) time, and the need for an intra-aortic balloon pump, a ventricular assist device, extracorporeal membrane oxygenation, or red blood cell transfusion (2, 3). Postoperative risk factors include the length of pediatric intensive care unit (PICU) or hospital stay, complications such as cardiac surgery-associated acute kidney injury (CS-AKI), nosocomial infections, septic shock, cardiogenic shock, and heart failure (4-6).
Perioperative bleeding, arrhythmia, myocardial dysfunction, pneumothorax, and pulmonary hypertension can also affect the prognosis of cardiac surgery and the duration of hospital and ICU stay (7). Among the intraoperative factors, a longer CPB time is linked with increased postoperative complications (8). As known, CPB generates an inflammatory condition, which can be reflected by the neutrophil-to-lymphocyte ratio (NLR). Prognosis can be predicted based on this ratio, along with the platelet count (9).
2. Objectives
In this study, we evaluated the relationship between mortality in CHD children and Risk Adjustment for Congenital Heart Surgery (RACHS-1) score, age, weight, type of the congenital defect, duration of ICU stay, duration of CPB, aortic cross-clamping (XCT) time, and postoperative platelet count and NLR following cardiac surgery.
3. Methods
Our retrospective, cross-sectional study was conducted at a tertiary referral hospital in Tehran, Iran, between March 2018 and March 2019. A total of 275 patients younger than 18 years who underwent open heart surgery admitted to the open heart surgery intensive care unit (OH-ICU) were evaluated. The data were obtained from their medical records in January 2021. The same well-trained and experienced surgical team performed all surgeries. Inclusion criteria included patients under 18 who had undergone open heart surgery and were admitted to the OH-ICU. Patients with prior thrombocytopenia, malignancies, or chemo/radiotherapy exposure were excluded.
The collected data included age (months), weight (kg), CHD type, RACHS-1, duration of admission in the OH-ICU (days), duration of CPB and XCT (minutes), postoperative platelet count and NLR (first day after surgery), and mortality. Data were analyzed using Stata v. 14.2 software and reported as mean ± standard deviation (SD) or frequency (percentage). A P value ≤ 0.05 was considered significant. This study was approved by the Institutional Research Ethics Committee of the Children's Medical Center of Tehran University of Medical Sciences (IR.TUMS.CHMC.REC.1398.096).
4. Results
This study included 275 CHD patients who underwent cardiac surgery. The mean age and weight were 32.54 ± 37.4 months and 11.01 ± 8.43 kg, respectively. The patients ranged in age from 0.16 to 204 months. The mean CPB and XCT durations were 109.3 ± 75.23 and 77.54 ± 57.75 minutes, respectively. The mean postoperative NLR was 3.95 ± 2.71 (Table 1).
| Variables | Mean ± SD | Range |
|---|---|---|
| Age (mo) | 32.54 ± 37.4 | 0.16 – 204 |
| Weight (kg) | 11.01 ± 8.43 | 1.2 – 55.5 |
| Duration of hospitalization (days) | 6.95 ± 7.73 | 2 – 65 |
| CPB time (min) | 109.3 ± 75.23 | 0 – 350 |
| XCT time (min) | 77.54 ± 57.75 | 0 – 291 |
| Neutrophil-to-lymphocyte ratio | 3.95 ± 2.71 | 0.14 – 18.77 |
| Platelet count (x109/L) | 180.48 ± 100.52 | 35 – 752 |
Abbreviations: CPB, cardiopulmonary bypass; XCT, aortic cross-clamping.
The prevalence of thrombocytopenia was 44.37%. The patients were categorized by their platelet count into four groups to assess the impact of the extent of thrombocytopenia on mortality (Table 2).
| Group | No. (%) |
|---|---|
| Severe thrombocytopenia (< 50 x 109/L) | 6 (2.18) |
| Moderate thrombocytopenia (50 – 100 x 109/L) | 45 (16.36) |
| Mild thrombocytopenia (100 – 150 x 109/L) | 71 (25.81) |
| Non-thrombocytopenic (≥ 150 x 109/L) | 153 (55.63) |
Nine deaths (3.2%; 95% confidence interval: 1.5 – 6.1%) were reported. Although the relationship between the specific disease type and mortality was significant (P = 0.02), the CHDs could only be broadly categorized due to the wide variation in abnormalities (Table 3). Therefore, the distribution of the RACHS-1 score (Risk Adjustment for Congenital Heart Surgery) was evaluated. A direct linear relationship existed between the RACHS-1 score and mortality but was insignificant (P = 0.23). The mean NLR was higher in survival patients (4.08 vs. 2.87) (Table 4).
| Disease | Survived (n = 268) | Deceased (n = 9) |
|---|---|---|
| Non-cyanotic | 150 (98) | 3 (2) |
| ALCAPA (anomalous left coronary artery from the pulmonary artery) | 1 (100) | 0 (0) |
| AORPA (Anomalous origin of the right pulmonary artery from the ascending aorta) | 1 (100) | 0 (0) |
| APW (aortopulmonary window) | 1 (100) | 0 (0) |
| AS (aortic stenosis) | 8 (100) | 0 (0) |
| ASD2 (atrial septal defect secundum) | 21 (100) | 0 (0) |
| AVSD (atrioventricular septal defect) | 11 (100) | 0 (0) |
| CoA (coarctation of the aorta) | 16 (84.2) | 3 (15.8) |
| MI (mitral insufficiency) | 2 (100) | 0 (0) |
| PDA (patent ductus arteriosus) | 6 (100) | 0 (0) |
| PS (pulmonary stenosis) | 11 (100) | 0 (0) |
| Sinus venosus ASD | 1 (100) | 0 (0) |
| VSD (ventricular septal defect) | 71 (100) | 0 (0) |
| Cyanotic | 116 (95) | 6 (5) |
| Ebstein anomaly | 1 (100) | 0 (0) |
| Single ventricle | 16 (100) | 0 (0) |
| PAPVC (partial anomalous pulmonary venous connection) | 5 (83.3) | 1 (16.7) |
| PAVSD (pulmonary atresia with ventricular septal defect) | 5 (83.3) | 1 (16.7) |
| TAPVR (total anomalous pulmonary venous return) | 10 (90.9) | 1 (9.1) |
| TAT (tricuspid atresia) | 3 (100) | 0 (0) |
| TGA IVS (transposition of great arteries with intact ventricular septum) | 10 (100) | 0 (0) |
| TGA VSD (transposition of great arteries with ventricular septal defect) | 16 (100) | 0 (0) |
| ToF (tetralogy of fallot) | 49 (96.1) | 2 (3.9) |
| Truncus arteriosus | 1 (50) | 1 (50) |
| Total | 266 (96.7) | 9 (3.3) |
| P-value b | 0.02 | |
a Values are expressed as No. (%).
b The P value was calculated using the t-test or Pearson's chi-square test.
| Variables | Survived | Deceased | P-Value b |
|---|---|---|---|
| Demographics | |||
| Age (mo) | 32.82 ± 37.37 | 23.13 ± 41.54 | 0.44 |
| Weight (kg) | 11.18 ± 8.44 | 5.9 ± 7.28 | 0.06 |
| Clinical characteristics | |||
| Duration of hospitalization (days) | 6.66 ± 6.81 | 15.78 ± 20.64 | 0.22 |
| CPB time (min) | 108.64 ± 73.53 | 137.44 ± 120.59 | 0.25 |
| XCT time (min) | 77.28 ± 57.57) | 88 ± 67.84 | 0.58 |
| Neutrophil-to-lymphocyte ratio | 4.08 ± 2.95 | 2.87 ± 1.25 | 0.22 |
| Platelet count (x109/L) | 181.08 ± 100.88 | 142.11 ± 90.68 | 0.24 |
| Disease category | 0.17 | ||
| Cyanotic | 116 (95.08) | 6 (4.92) | |
| Non-cyanotic | 150 (98.03) | 3 (1.97) | |
| RACHS-1 level | 0.23 | ||
| 1 | 31 (100) | 0 (0) | |
| 2 | 143 (97.9) | 3 (2.1) | |
| 3 | 81 (94.2) | 5 (5.8) | |
| 4 | 13 (92.9) | 1 (7.1) |
Abbreviations: CPB, cardiopulmonary bypass; XCT, aortic cross-clamping; RACHS-1, risk adjustment for congenital heart surgery.
a Values are expressed as Mean ± SD or No. (%).
b The P value was calculated using the t-test or Pearson's chi-square test.
The patients' age and weight had no significant relationship with mortality (P = 0.44 and 0.06, respectively). The relationship between thrombocytopenia and mortality was not significant (P = 0.24), as was the relationship between NLR and mortality (P = 0.22) (Table 4).
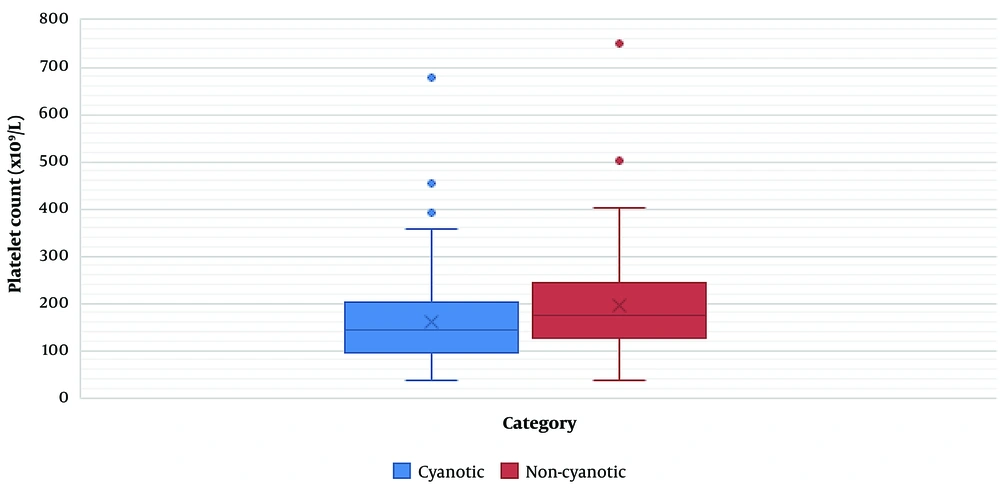
Of 275 cases, 153 (55.63 %) were non-cyanotic, and the remaining (n = 122; 44.37 %) had cyanotic heart diseases. We found no significant relationship between mortality and disease category (cyanotic or non-cyanotic) (P = 0.17) (Table 4). The CPB time, XCT time, and platelet count were significantly related to the disease category. The mean CPB (81.66 min) and XCT (58.58 min) were lower in non-cyanotic patients than in cyanotic patients (143.97 and 101.31 min, respectively) (P < 0.0001). The mean platelet count was significantly higher in non-cyanotic patients (196.65 x 109/L) than in cyanotic patients (mean = 160.19 x109 /L) (P = 0.0003) (Table 5) (Figure 1).
| Variables | Non-cyanotic | Cyanotic | P-Value b |
|---|---|---|---|
| CPB time (min) | 81.66 ± 60.46 | 143.97 ± 78.05 | < 0.0001 |
| XCT time (min) | 58.58 ± 45.09 | 101.31 ± 63.26 | < 0.0001 |
| Platelet count (x109/L) | 196.65 ± 104.2 | 160.19 ± 92.64 | 0.0003 |
Abbreviations: CPB, cardiopulmonary bypass; XCT, aortic cross-clamping.
a Values are expressed as Mean ± SD or No. (%).
b The P-value was calculated using the Mann-Whitney U test.
5. Discussion
This study explored potential risk factors associated with the postoperative mortality of children undergoing cardiac surgery for congenital heart disease. The studied variables included age, body weight, duration of hospitalization, intraoperative CPB and XCT time, and NLR, as well as platelet count on the first day after the surgery.
As CPB initiates an inflammatory response, the NLR as an inflammation marker might predict the outcome of cardiac surgery. In one study, a higher NLR was linked with unfavorable outcomes and longer postoperative mechanical ventilation (9). A low platelet count after cardiac surgery might also indicate the prognosis. In the ICU, thrombocytopenia signifies organ dysfunction and is related to increased mortality. Thrombocytopenia may occur because of the inflammatory response to CPB, the activation of inflammatory cytokines, and autoimmune reactions (9). A higher RACHS-1 level is also directly related to increased postoperative complications (10). In our study, the RACHS-1 score was not significantly related to mortality. Our sample size of patients with different CHDs was likely insufficient to find a statistically significant relationship. Also, the types of surgeries performed and the variations in surgeons' skills could explain why we found no link between the RACHS-1 score and mortality.
Our study found lower age at surgery was not significantly associated with mortality following pediatric heart surgery. However, the deceased patients had lower weights than the survivors. This contrasts with other studies (7). Elassal et al. expressed that weight might not be associated with mortality following cardiac surgery (11). Variations in the skills and case selection of surgeons may explain such discrepancies.
Eckersley et al. reported 18% mortality in critical versus 8% in non-critical cases and 12% of death with early diagnosis versus 29% with late diagnosis (12). Among 37,386 procedures recorded in the World Database for Pediatrics and Congenital Heart Surgery (WDPCHS) from 39 centers across 22 countries, 93% required CPB (median 84 minutes), and 97% required XCT (median 52 minutes). The overall hospital mortality was 4.3% (1,445 cases) (13). In a study on 1,155 cardiac operations by Elassal et al., the median length of stay was 24 days, and the postoperative hospital mortality rate was 11%. Among 136 procedures performed on neonates, 110 (80.9%) needed CPB. The median CPB and XCT times were 81 and 51 minutes, respectively. It was concluded that age, weight, RACHS-1 score, and need for CPB had no significant relationship with the mortality rates (11).
Some studies have mentioned that CPB can initiate the inflammatory response (9) and linked a longer CPB time and more complicated surgery (higher RACHS-1) with more postoperative complications (10). The different activities of the surgical centers can justify the contrasting results of the studies. For instance, our patients did not have hypoplastic left heart syndrome or other very complex CHDs.
Different risk assessment models such as the RACHS-1, the Society of Thoracic Surgeons–European Association for Cardio-Thoracic Surgery (STS-EACTS) mortality score, the STS morbidity score, and the Aristotle Basic score have been used to predict outcomes following pediatric cardiac surgeries (14-16). We used the RACHS-1 model introduced by Jenkins et al. in 2002. It is a consensus-based method for risk adjustment for in-hospital mortality among patients younger than 18 years following cardiac surgery for CHD (16). The grouping of subjects in this model can help predict and improve procedural outcomes. In our study, RACHS-1 scores were not significantly associated with mortality.
Since CHDs are a heterogeneous group of severe disorders with different surgical and nonsurgical outcomes (10, 17), each patient with a specific anomaly should be examined individually. Our study found a significant relationship between the type of anomaly and outcomes. These findings are based on the subjects' age and duration of CPB. Still, we did not find a significant correlation with the complexity of the procedure, which could be due to the low study volume and heterogenicity of the anomalies. The different skill and experience levels of the surgeons may also affect the outcome of the surgery. Such factors may explain why we failed to find a relationship between the RACHS-1 score and the outcome of surgery.
Li et al.'s study on 311 children aged one month to 18 years undergoing pediatric cardiac surgery described the effect of CPB time and length of hospital stay on prognosis and complications. They concluded that CPB time could be a marker for subjects' complexity. Cases with more extended hospital stays and longer CPB times were at increased risk of complications such as Acute kidney injury (AKI) (18). We found no significant relationship between CPB and XCT durations and mortality. However, the deceased patients had higher CPB times. We also showed that the CPB time was significantly higher in cyanotic CHDs than in noncyanotic CHDs. Further studies with larger sample sizes and more surgeons should investigate these variables' effects.
Postoperative thrombocytopenia can be attributed to the innate defects and anomalies that reduce platelet production or augment platelet destruction; platelet drops are also linked with CPB and cardiac surgery-induced AKI (19). Postoperative platelet depletion and thrombocytopenia are independently associated with postoperative mortality and complications such as AKI, infection, and extended hospital stays, worsening the mortality rate (20). Thrombocytopenia is a marker of organ failure or autoimmune platelet destruction caused by the CPB-induced inflammation response (9). However, in our study, a lower platelet count on the first postoperative day was not associated with increased mortality. Further studies with larger samples should be performed to explain the relationship between blood cell components and the outcome.
As a measure of systemic inflammation, NLR is significantly associated with chronic conditions such as diabetes mellitus, hypertension, and hyperlipidemia. A positive correlation exists between the NLR and standard inflammatory parameters, including C-reaction protein, erythrocyte sedimentation rate, and SLE Disease Activity Index (SLEDAI) scores (21-23). Also, NLR is considered a biomarker for cardiovascular disease (CVD), and subjects with higher NLR are at greater risk for CVD and stroke. Subjects with CVD and stroke with higher NLR have a worse prognosis and higher mortality rate (24, 25). Increased NLRs have been linked with a poor prognosis of cardiac surgery in some studies (9, 26). Although NLR was not significantly associated with mortality in our patients, the deceased patients did have lower NLRs. This finding is in contrast with other research. Apart from the different sample sizes and populations, preoperative and postoperative treatment with corticosteroids and the waiting time for surgery might also explain discrepancies between the studies.
5.1. Limitations
We conducted a single-center, retrospective study of a heterogeneous group of diseases; hence, limits in access to other potentially important data could have affected our findings. Furthermore, the number of subjects for each anomaly and each category of anomalies was limited.
5.2. Conclusions
Mortality following cardiac surgery did not significantly correlate with the age, weight, CPB time, NLR, platelet count (first postoperative day), and RACHS-1 scores of CHD patients. However, the deceased patients had a lower weight, younger age, longer CPB time, lower platelet count, and lower NLR on the first postoperative day. Noncyanotic CHD patients had a significantly higher platelet count than cyanotic ones on the first operative day. The mortality rate following cardiac surgery was not significantly different between cyanotic and noncyanotic CHDs, though cyanotic postoperative mortality was greater. More research with larger sample sizes and more surgeons should be performed in various regions to investigate the relationship between different factors and the outcome of surgery for CHDs.