Abstract
Keywords
1. Background
Fanconi syndrome (FS) is a rare metabolic disease identified by dysfunction of the renal proximal tubules. The low reabsorption capacity of the proximal tubules results in the abnormal urinary excretion of several metabolites, including phosphate, glucose, amino acids, uric acid, and various ions. Other biochemical findings include hypokalemia, hypouricemia, and metabolic acidosis (1, 2). This syndrome is highly associated with osteomalacia, osteoporosis, muscle weakness, and severe bone pain. The production of monoclonal light chains, especially kappa chains, which have toxic effects on the renal tubules, is also reported in these patients (3).
FS may be a genetic disease with primary or secondary causes, or may be acquired. A missense mutation in sodium phosphate cotransporter (NaPi-II) of the proximal tubular apical membrane accounts for primary FS. This syndrome may also develop secondary to systemic disease. The genetic and acquired causes of FS are indicated in Table 1. The causes of FS may also lead to renal tubular acidosis. Light-chain-associated FS is the most frequent among the acquired factors (4).
| Primary | Missense Mutation of NaPi-II Cotransporter |
|---|---|
| Cystinosis | |
| Tyrosinemia | |
| Hereditary fructose intolerance | |
| Galactosemia | |
| Glycogen storage disease (type I) | |
| Mitochondrial disorders | |
| Wilson’s disease | |
| Lowe’s syndrome | |
| Den’s disease | |
| Fanconi-Bickel syndrome | |
| Drugs (tenofovir, ifosfamide, cisplatin, valproic acid, aminoglycosides, cidofovir) | |
| Heavy metals | |
| Multiple myeloma | |
| Amyloidosis | |
| Vitamin D deficiency | |
| Renal transplantation | |
| Paroxysmal nocturnal hemoglobinuria |
Dysglobulinemia is a feature of FS, and some studies in the literature report FS in association with myeloma, amyloidosis, and Bence-Jones proteinuria (5). In this study, we report a unique case of FS that was complicated by bilineal acute leukemia with myeloid and T-lymphoid lineages.
2. Case Presentation
An 8-year-old boy was admitted to our center complaining of pallor, anemia, asthenia, and recurrent infections in January 2014. The patient was the first child of a family with three children. Physical examination revealed severe growth failure, with a weight of 12 kg weight, height of 97 cm, and head circumference of 46 cm. Hepatosplenomegaly was observed with a palpable liver and spleen 3 cm and 2 cm below the costal margin, respectively. On abdominal ultrasound, the sizes of the liver and spleen were 95 mm and 86 × 40 mm, respectively, and an echogenic focus in the left kidney was also detected. The patient presented with genu valgum and was not able to walk easily. Bilateral cataracts and lens opacity were found, with 6/10 and 7/10 visual acuity for the right and left eyes, respectively. Wrist radiography showed widening, fraying, and cupping, all suggestive of rickets, and bone age was 3.5 years. Complete blood count (Sysmex KX 21N, Japan) demonstrated a white blood cell count of 4.2 × 109/L with 87% lymphocytes, a hemoglobin level of 4.9 g/dL, mean corpuscular volume of 74 fl, and a platelet count of 73×109/L. The erythrocyte sedimentation rate was 104 mm/hr. The ferritin level was extremely high (2250 ng/mL; normal range is 12 - 142 for children). Venous blood gas analysis (ABL 735; Radiometer, Copenhagen, Denmark) was as follows: pH 7.30, pCO2 29 mmHg, HCO3 13 Meq/L, BE 10 Meq. Liver function tests, thyroid function tests, and evaluation for celiac disease by tissue transglutaminase IgA antibody (TTG IgA) were normal. The results of biochemical testing (Hitachi 917, Hitachi, Japan) are summarized in Table 2.
Biochemical Test Results for the Patient
| Test | Result | Normal Values | Low/Normal or High According to Normal Values |
|---|---|---|---|
| 91 mg/dL | 70 - 110 mg/dL | Normal | |
| 9 mg/dL | 7 - 18 mg/dL | Normal | |
| 0.7 mg/dL | 0.6 - 1.2 mg/dL | Normal | |
| 137 mEq/L | 135 - 145 mEq/L | Normal | |
| 3.3 mEq/L | 3.5 - 5.0 mEq/L | Low | |
| 8.7 mg/dL | 8.4 - 10.2 mg/dL | Normal | |
| 1.7 mg/dL | 3.0 - 8.2 mg/dL | Low | |
| 463 U/L | 45 - 90 U/L | High | |
| 1.1 mg/dL | 3.0 - 4.5 mg/dL | Low |
Immunologic analysis revealed a low IgG level (6 g/L, normal 6.46 - 14.51) but high IgM (2.7 g/L, normal 0.55 - 2.32) and IgA (2.3 g/L, normal 0.28 - 2.22). C-reactive protein was 3+ on the latex agglutination test. Urine analysis showed glucosuria and proteinuria, with a pH of 5. According to the physical examination results, clinical manifestations, radiologic findings, and laboratory results, FS was diagnosed. On microscopic examination of the bone marrow aspirate (BMA), two different populations of blasts were observed (Figure 1). Subsequent flow cytometric analysis (Partec, CyFlow® Space) revealed 98% blasts with positivity for CD2, cytoplasmic CD3, CD7, TdT, CD13, CD34, CD117, and myeloperoxidase. Common chromosomal translocations of acute leukemia, including t(4,11), t(12,21), t(1,19), and t(9,22) by the PCR method were all negative. According to the world health organization (WHO) 2008 classification (6), these findings are indicative of a bilineal acute leukemia with myeloid and T-lymphoid lineages.
Bone Marrow Aspiration Showing Two Different Populations of Blasts
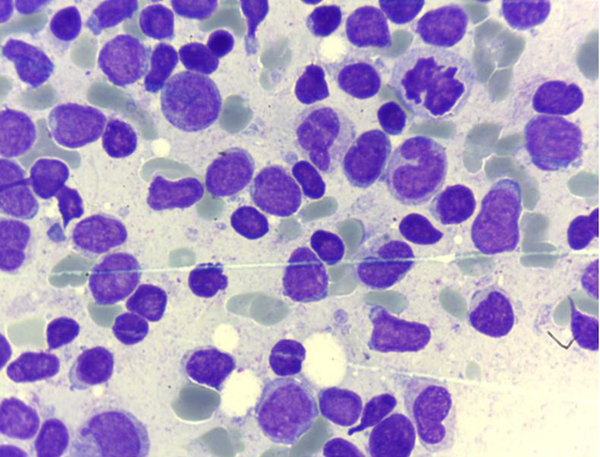
Cerebrospinal fluid analysis and cytology was normal. The patient received Joulie’s solution for the FS, and a combined chemotherapy regimen that was effective for both acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL) (modified by the St. Jude XIII-B high risk protocol) (7). Despite supportive treatments and administration of wide spectrum antibiotics, the patient died at the end of the induction phase of chemotherapy due to sepsis and disseminated intravascular coagulation (DIC). Further examinations of family members revealed that the patient’s 4-year-old sister was similarly diagnosed with FS. The third child of this family, a newborn baby, was not investigated.
3. Discussion
Given that dysglobulinemia is a feature of FS, it may be commonly associated with myeloma, amyloidosis, and Bence-Jones proteinuria (5). Ma et al. suggested acquired FS as a complication of monoclonal gammopathy in adults (8). Messiaen et al. reported several cases of multiple myeloma that subsequently developed FS (9). Rochman et al. reported a case of FS as the result of chronic lymphocytic leukemia (10). There is also a study by Bridoux et al. reporting the development of FS in a patient with Waldenström’s macroglobulinemia (11). The authors suggested that the accumulation of kappa light chains may have played a significant role in the development of FS in the reported patients (9, 11). Another study reported a case with chronic myelogenic leukemia, who developed partial FS after receiving imatinib mesylate (Gleevec) (12).
Considering the familial occurrence and the childhood onset of FS in our patient, an inherited background is probable. The unusual event in our patient is the development of an almost rare acute leukemia, a condition which has not been reported before.
Although the majority of acute leukemias can be classified as myeloid, B lymphoid, or T lymphoid lineages through morphology, immunophenotyping, cytogenetic, and molecular analyses, there are also rare cases that manifest the characteristics of at least two lineages and account for 2% - 5% of all acute leukemias (13). Generally, two main types can be located in this group: biphenotypic leukemia and bilineal leukemia. The former refers to leukemias in which divergent features present in a single blast population, while the latter describes leukemias with two different populations of blasts (14). In the WHO 2008 classification, both types are termed as mixed-phenotype acute leukemia (MPAL) (6). The lineage-specific markers suggested by the WHO 2008 are listed in Table 3. Accordingly, positivity of myeloperoxidase and cytoplasmic CD3 in either a single or distinct population of blasts (as was found in our patient) are sufficient for a diagnosis of MPAL with T/myeloid differentiation. In this classification, MPAL with BCR-ABL1 fusion or MLL gene rearrangements with the most common partner gene, AF4 at the 4q21 location, have been regarded as separate entities (6).
In our patient, there was no evidence of t(9,22)(q34;q11), which leads to BCR-ABL1 fusion, or of t(4,11)(q21;q23), which most commonly involves the MLL gene. Meeting the WHO 2008 criteria, MPAL T/myeloid, not otherwise specified was the diagnosis in our patient.
| Lineage | Markers |
|---|---|
| Myeloperoxidase Or Monocytic differentiation (at least two of the following: non-specific esterase, CD11c, CD14, CD64, lysozyme) | |
| Cytoplasmic CD3 or Surface CD3 | |
| Strong CD19 and at least one of the following with strong expression: CD79a, cytoplasmic CD22, or CD10 or Weak CD19 and at least two of the following with strong expression: CD79a, cytoplasmic CD22, or CD10 |
Like other acute leukemias, the clinical presentations of MPAL are mainly a result of BM failure, and according to the literature, these include fatigue, infections, and bleeding disorders (6). Hepatosplenomegaly, thrombocytopenia, and severe anemia with high levels of ferritin in our patient were supportive of BM failure, but no bleeding tendency was found. According to the published studies, the outcome of MPAL is generally poor (15). The study by Lee et al. indicated that patients with myeloid/T-lymphoid have an even lower chance of survival than patients affected by the rest of the MPALs (16). Currently, there is no agreement on the treatment of MPAL patients, but a chemotherapy regimen effective for both AML and ALL has been suggested (6). However, Killick et al. noted that such a therapeutic protocol is associated with a high rate of early death in these patients (17). Consistent with the therapeutic strategy elected for in the study by Al-Seraihy et al. we chose the modified St. Jude XIII-B high-risk protocol for our patient, since they suggested that this type of therapy is effective at gaining and maintaining complete remission (18).
References
-
1.
Kinoshita-Katahashi N, Fukasawa H, Ishigaki S, Isobe S, Imokawa S, Fujigaki Y, et al. Acquired Fanconi syndrome in patients with Legionella pneumonia. BMC Nephrol. 2013;14:171. [PubMed ID: 23915094]. https://doi.org/10.1186/1471-2369-14-171.
-
2.
Ghiculescu RA, Kubler PA. Aminoglycoside-associated Fanconi syndrome. Am J Kidney Dis. 2006;48(6):e89-93. [PubMed ID: 17162140]. https://doi.org/10.1053/j.ajkd.2006.08.009.
-
3.
Pham T, Furno-Steib S, Daumen-Legre V, Acquaviva PC, Lafforgue P. Bilateral symmetric polyarthralgia revealing Fanconi's syndrome. Joint Bone Spine. 2002;69(2):209-13. [PubMed ID: 12027314].
-
4.
Haque SK, Ariceta G, Batlle D. Proximal renal tubular acidosis: a not so rare disorder of multiple etiologies. Nephrol Dial Transplant. 2012;27(12):4273-87. [PubMed ID: 23235953]. https://doi.org/10.1093/ndt/gfs493.
-
5.
Maldonado JE, Velosa JA, Kyle RA, Wagoner RD, Holley KE, Salassa RM. Fanconi syndrome in adults. A manifestation of a latent form of myeloma. Am J Med. 1975;58(3):354-64. [PubMed ID: 163583].
-
6.
Weinberg OK, Arber DA. Mixed-phenotype acute leukemia: historical overview and a new definition. Leukemia. 2010;24(11):1844-51. [PubMed ID: 20844566]. https://doi.org/10.1038/leu.2010.202.
-
7.
Pui CH, Sandlund JT, Pei D, Campana D, Rivera GK, Ribeiro RC, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Total Therapy Study XIIIB at St Jude Children's Research Hospital. Blood. 2004;104(9):2690-6. [PubMed ID: 15251979]. https://doi.org/10.1182/blood-2004-04-1616.
-
8.
Ma CX, Lacy MQ, Rompala JF, Dispenzieri A, Rajkumar SV, Greipp PR, et al. Acquired Fanconi syndrome is an indolent disorder in the absence of overt multiple myeloma. Blood. 2004;104(1):40-2. [PubMed ID: 15010372]. https://doi.org/10.1182/blood-2003-10-3400.
-
9.
Messiaen T, Deret S, Mougenot B, Bridoux F, Dequiedt P, Dion JJ, et al. Adult Fanconi syndrome secondary to light chain gammopathy. Clinicopathologic heterogeneity and unusual features in 11 patients. Medicine (Baltimore). 2000;79(3):135-54. [PubMed ID: 10844934].
-
10.
Rochman J, Lichtig C, Osterweill D, Tatarsky I, Eidelman S. ASdult Fanconi's syndrome with renal tubular acidosis in association with renal amyloidosis: occurrence in a patient with chronic lymphocytic leukemia. Arch Intern Med. 1980;140(10):1361-3. [PubMed ID: 6775610].
-
11.
Bridoux F, Sirac C, Hugue V, Decourt C, Thierry A, Quellard N, et al. Fanconi's syndrome induced by a monoclonal Vkappa3 light chain in Waldenstrom's macroglobulinemia. Am J Kidney Dis. 2005;45(4):749-57. [PubMed ID: 15806478].
-
12.
Francois H, Coppo P, Hayman JP, Fouqueray B, Mougenot B, Ronco P. Partial fanconi syndrome induced by imatinib therapy: a novel cause of urinary phosphate loss. Am J Kidney Dis. 2008;51(2):298-301. [PubMed ID: 18215707]. https://doi.org/10.1053/j.ajkd.2007.10.039.
-
13.
Yan L, Ping N, Zhu M, Sun A, Xue Y, Ruan C, et al. Clinical, immunophenotypic, cytogenetic, and molecular genetic features in 117 adult patients with mixed-phenotype acute leukemia defined by WHO-2008 classification. Haematologica. 2012;97(11):1708-12. [PubMed ID: 22581002]. https://doi.org/10.3324/haematol.2012.064485.
-
14.
Bleahu ID, Vladasel R, Gheorghe A. A special case of acute leukemia in childhood. J Med Life. 2011;4(3):297-301. [PubMed ID: 22567056].
-
15.
Weir EG, Ali Ansari-Lari M, Batista DA, Griffin CA, Fuller S, Smith BD, et al. Acute bilineal leukemia: a rare disease with poor outcome. Leukemia. 2007;21(11):2264-70. [PubMed ID: 17611554]. https://doi.org/10.1038/sj.leu.2404848.
-
16.
Lee JH, Min YH, Chung CW, Kim BK, Yoon HJ, Jo DY, et al. Prognostic implications of the immunophenotype in biphenotypic acute leukemia. Leuk Lymphoma. 2008;49(4):700-9. [PubMed ID: 18398737]. https://doi.org/10.1080/10428190701843247.
-
17.
Killick S, Matutes E, Powles RL, Hamblin M, Swansbury J, Treleaven JG, et al. Outcome of biphenotypic acute leukemia. Haematologica. 1999;84(8):699-706. [PubMed ID: 10457405].
-
18.
Al-Seraihy AS, Owaidah TM, Ayas M, El-Solh H, Al-Mahr M, Al-Ahmari A, et al. Clinical characteristics and outcome of children with biphenotypic acute leukemia. Haematologica. 2009;94(12):1682-90. [PubMed ID: 19713227]. https://doi.org/10.3324/haematol.2009.009282.