Abstract
Background:
The family of heat shock proteins (HSPs) contains several members that regulate the cell response to any hazardous factors to protect protein structure. The aim of the study was to determine whether serum levels of HSPs increase in children with IgA nephropathy/Immunoglobulin A - associated vasculitis nephritis (IgAN/IgAVN) and to assess their relationship with the prognostic factors.Patients and Methods:
Twenty - nine patients with IgAN/IgAVN, 41 patients with idiopathic nephrotic syndrome (INS) as a patient control group, and 24 healthy controls were enrolled in the study. Renal function was normal in all participants. Serum levels of HSP27, HSP40, HSP60, HSP70, and HSP90 were measured using an enzyme - linked immunosorbent assay (ELISA).Results:
Median serum levels of HSP27, HSP40, HSP60, HSP70 and HSP90 were significantly higher in the IgAN/IgAVN group than in the INS group and controls (P < 0.05). Among these HSPs, only HSP60 had no overlap between patients and controls. Serum HSP40, HSP60, HSP70 levels were significantly higher in the patients with INS than in controls (P < 0.05), whereas HSP27 and HSP90 were not different. HSP27 was significantly higher in patients with proteinuria in the IgAN/IgAVN group than in patients with no proteinuria (P = 0.045). None of these HSPs was related to the presence of crescent and severity of proteinuria in the IgAN/IgAVN group.Conclusions:
Our results demonstrated that serum levels of HSP27, 40, 60, 70, 90 increased in the patients with IgAN/IgAVN considering HSPs might have a role in pathogenesis of the disease. Among these HSPs, HSP60 was a prominent marker because it had no overlap between patients and controls. Explaining the role of these HSPs in IgAN/IgAVN may pave the way for future treatment.Keywords
1. Background
Heat shock proteins (HSPs) are intracellular chaperones that are produced under stress conditions (1). HSPs are categorized to their molecular weight such as small HSPs (16-40 kDa), HSP60 (60 kDa), HSP70 (70 kDa), HSP90 (90 kDa) (1). As an intracellular chaperon, their main function is to provide cell survival via protecting protein structure (1). Some experimental and clinical studies show that some HSPs have an effect in glomerular disease (2). Razzaque et al (3) demonstrated that HSP47 expression was related to collagen production in diabetic nephropathy and immunoglobulin A nephropathy (IgAN). Urine HSP70 levels were found to be elevated in chronic glomerulonephritis and these higher levels of HSP70 were associated with disease prognosis (4). Lang et al. (5) demonstrated that injection of HSP60 aggravated global glomerular necrosis and tubulointerstitial damage in experimental immune-mediated nephritis. Also, there is some evidence that HSP25/27 has a role in the maintenance of podocyte structure in experimental studies of nephrotic syndrome (NS) (6).
Immunoglobulin A nephropathy is the most common primary glomerulonephritis in the adult population, it is also a common glomerulonephritis in children (7). IgAN has a wide spectrum of clinical presentations including microscopic hematuria, and a different range of proteinuria. End - stage renal disease (ESRD) develops in up to half of patients within 20 years (7-9). Immunoglobulin A - associated vasculitis is one of the most common vasculitides in children (10). Renal involvement of this disease is mostly mild; however, a small percentage of patients have a large amount of proteinuria or nephrotic syndrome and impairment of renal function (10). ESRD may develop in 1-2% of patients with immunoglobulin A - associated vasculitis nephritis (IgAVN) (11). Also, IgAVN has the same immunohistologic features as IgAN (11).
It has been considered that HSPs may involve the pathogenesis of glomerulonephritis and may aggravate the disease or some of these HSPs may have a beneficial role in the prognosis of NS (2). Therefore, we aimed to determine serum levels of HSPs in children with IgAN/IgAVN and to assess their relationship with prognostic factors.
2. Methods
2.1. Patients and Methods
Twenty - nine patients with IgAN/IgAVN, 41 patients with idiopathic nephrotic syndrome (INS) as a patient control group, and 24 healthy controls were enrolled in the study. Sex distribution was not different among the three groups (P > 0.005). Age distribution of the INS and control groups was not significantly different (P > 0.05) although the mean age was higher in the IgAN/IgAVN group than in the other groups (P = 0.002). Approval for this study was obtained from the Local Ethics Committee (Project No. 2015/367). Informed consent was obtained from the parents of all participants.
2.2. Immunoglobulin A Nephropathy/Immunoglobulin A - associated Vasculitis Nephritis Group
Twenty - nine patients with biopsy - proven IgAN/IgAVN (7 female, 22 male) were enrolled in the study. Seventeen had IgAN (58.6%) and 12 had IgAVN (41.4%). The mean age of the IgAN/IgAVN group was 13.19 ± 4.57 years (range, 3.5 - 19 years). IgAN/IgAVN were diagnosed based on clinical, laboratory, and immunohistopathologic findings. Mesangial hypercellularity with predominant IgA deposition in mesangium and/or capillary wall was described as IgAN in patients without systemic involvement. European League Against Rheumatism/ Paediatric Rheumatology European Society (EULAR/PReS) consensus criteria were used for the diagnosis of IgAVN (12). The mean duration between kidney biopsy and sampling was 35.54 ± 32.12 months (range, 0 - 111 months). MEST [mesangial (M), endocapillary (E) hypercellularity, segmental sclerosis (S) and interstitial fibrosis/tubular atrophy (T)] score was evaluated in accordance with the Oxford classification (13). Eleven patients had mesangial hypercellularity (M1), 13 had endocapillary hypercellularity (E1), 6 had segmental glomerulosclerosis (S1), and 2 had tubular atrophy (T1). Presence/absence of crescents and number of crescents was recorded. Cellular crescents were defined as extracapillary proliferation of cells in Bowman’s space with more than two cell layers (14). Crescent formation was detected in 11 (35.48%) patients. Ten patients were receiving angiotensin converting enzyme inhibitor (ACEi) and omega-3 fatty acids, 3 patients were receiving only omega-3 fatty acids, 2 patients were receiving methylprednisolone orally, ACEi and omega-3 fatty acid, and 2 patients were not receiving any treatment at the time of sampling in the IgAN group. Four patients were receiving methylprednisolone orally, 2 patients were receiving ACEi and omega-3 fatty acids, and 6 patients were not receiving any treatment at the time of sampling in the IgAVN group.
2.3. Idiopathic Nephrotic Syndrome Group
Forty - one (16 female, 25 male) children with idiopathic nephrotic syndrome (INS) were enrolled in the study as a patient control group. The mean age of the INS patients group was 9.2 ± 4.2 years (range, 3.5 - 17.8 years). Eighteen of the patients were in remission after discontinuation of steroid therapy, 11 were in remission but continued steroid treatment, and 12 had active proteinuria despite steroid use and other immune - suppressive treatments. Four patients had been receiving cyclosporine and one patient had been receiving mycophenolate mofetil; these 5 patients were diagnosed as having focal segmental glomerulosclerosis (FSGS) in active proteinuria group. The remaining seven patients with active proteinuria had a new episode of nephrotic syndrome and still had proteinuria despite initiation of steroid. INS was diagnosed in accordance with the criteria of the International Study for Kidney Diseases in Children (15). A kidney biopsy was performed in 15 patients in the INS group; 5 had FSGS and 10 had minimal change disease according to the renal biopsy.
2.4. Healthy Control Group
The healthy control group consisted of 24 healthy children (13 females, 11 males) with no kidney disease in their history or any acute and chronic diseases at the time of blood sampling. The mean age of the healthy control group was 10.69 ± 2.81 (4.5 - 15.8 years).
2.5. Physical Examination and Laboratory Measurements
Medical history and clinical findings of patients were recorded and physical examination was performed at the time of sampling. Patients with arterial blood pressure over 95th percentile for age and sex were accepted as having hypertension (16). Serum urea, creatinine, electrolytes, lipids, total protein, albumin, urinalysis, and 24 - hour urinary protein, serum HSP27, HSP40, HSP60, HSP70, and HSP90 were performed in all patients and controls. The mean estimated GFR (eGFR) was calculated using Schwartz formula (17). Renal function was normal in all patients except one with eGFR 70 mL/min/1.73 m2 in IgAN group and one with 77.9 mL/min/1.73 m2 in INS group. The presence of five or more red blood cells per high power field in a urine specimen was accepted as hematuria (18). Proteinuria was determined as a positive dipstick reading of ≥ 1 + and urinary excretion ≥ 4 mg/m2/hour in 24 - hour urine (18).
Venous blood samples were collected in tubes from the antecubital vein, following an overnight fasting. The tubes were centrifuged at 2000 g (10 min) to remove the serum. The blood and serum samples were analyzed within an hour. Serum urea, creatinine, total protein, albumin, and other biochemical parameters were determined using a Beckman Coulter AU5800 Clinical Chemistry, Dxl 800 Immunoassay Auto - Analyzer and commercial kits (Beckman Coulter, CA, USA). Urine protein levels were measured using Siemens BNII nephelometric system (Siemens Healthcare Diagnostics, USA) with reagents and protocols provided by the manufacturer.
Serum levels of HSP27, HSP40, HSP60, HSP70 and HSP90 were assessed by enzyme - linked immunosorbent assay (ELISA) technique. Serum HSP27, HSP40, HSP60, HSP70 and HSP90 were analyzed using Human Heat Shock Protein 27 (hSP-27), 40(hSP-40), 60 (hSP-60), 70(hSP-70), 90(hSP-90) ELISA Kit (Cat No: 23324, 20960, 20989, 20959, 20958 respectively) purchased from Bio Medical Assay (BMASSAY, Beijing, China) following the manufacturer’s instructions. Their levels were expressed as ng/mL. The detection and quantification limits were set at < 0.05 ng/ml for hSP-90, hSP-70, hSP-60, hSP-40 and < 0.1 ng/mL for hSP-27. The ELISA kit shows no cross reactivity with any of the cytokines. The intra-assay coefficient of variations (CV) of hSP-27, hSP-40, hSP-60, hSP-70, hSP-90 were 4.5%, 5.1%, 4.7%, 5.3%, 4.7% and the interassay CV were 7.1%, 8.3%, 6.9%, 6.2%, 7.8% respectively.
2.6. Statistical Methods
Statistical calculations were performed using NCSS 2007 for Windows. Besides standard descriptive statistical calculations (mean, standard deviation, median and IQR), the Kruskal - Wallis test was used for the comparison of groups, the Mann - Whitney U test was used for the comparison of two groups. The Chi - square test was performed during the evaluation of qualitative data. Pearson’s correlation test was used to determine the relationships between variables. The results were evaluated within a 95% confidence interval. Statistical significance level was established at P < 0.05.
3. Results
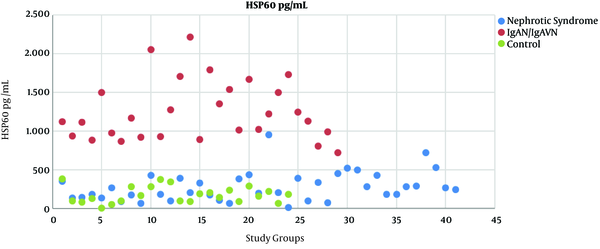
Median serum levels of HSP27, HSP40, HSP60, HSP70 and HSP90 were found significantly higher in patients with IgAN/IgAVN than in the INS group and controls (P < 0.05) (Table 1). Serum HSP40, HSP60, HSP70 levels were significantly higher in patients with INS than in the controls (P < 0.05); HSP27 and HSP90 were not different between two groups (Table 1). Among these HSPs, only HSP60 had no overlap between patients and controls (Figure 1).
Distribution of serum HSP60 levels of study group, HSP; Heat shock protein, IgAN; Immunoglobulin A Nephropathy, IgAVN; Immunoglobulin A - associated Vasculitis Nephritis.
| Healthy Control Group, (N = 24) | IgAN + IgAVN Group, (N = 29) | INS Group, (N = 41) | Pb | Pc | Pd | |
|---|---|---|---|---|---|---|
| HSP27 pg/mL | 502.10, (415.08-629.47) | 670.52, (524.56-1803.11) | 515.43, (405.96-784.56) | 0.0001 | 0.0001 | 0.359 |
| HSP40pg/mL | 231.90, (69.57-806.23) | 1044.47, (494.29-1824.30) | 378.36, (62.64-1216.75) | 0.0001 | 0.0001 | 0.033 |
| HSP60pg/mL | 164.69, (3.30-387.85) | 1123.65, (537.58-2220.07) | 246.31, (17.91-954.76) | 0.0001 | 0.0001 | 0.024 |
| HSP70pg/mL | 101.09, (58.65-257.10) | 325.72, (112.32-2650.72) | 117.31, (84.86-287.36) | 0.0001 | 0.0001 | 0.047 |
| HSP90pg/mL | 135.71, (15.15-1646.36) | 497.66, (134.38-2196.38) | 253.70, (34.35-2153.30) | 0.001 | 0.0001 | 0.065 |
There was no correlation between age and serum HSPlevels (P > 0.05). There was no difference between the patients with IgAN and IgAVN regarding serum levels of HSP27, HSP40, HSP60, HSP70, and HSP90 (P > 0.05) (Table 2). Median HSP27 was 707.018 (561.053-1803.117) in patients with proteinuria and 643.158 (524.561-1364.156) in patients without proteinuria in the IgAN/IgAVN group. HSP27 was significantly higher in patients with proteinuria in the IgAN/IgAVN group than in patients with no proteinuria (P = 0.045). There was no relationship between the presence of proteinuria and other HSPs. No HSPs were related to severity of proteinuria and presence of crescents in the IgAN/IgAVN group (P > 0.05). According to the MEST score, there was no difference between M1 and M0, E1 and E0, S1 and S0, T1 and T0 regarding all HSPs (P > 0.05).
The Comparison of Serum Heat Shock Protein Levels of the IgAN and IgAVN
| IgAN | IgAVN | P | |
|---|---|---|---|
| HSP27 (pg/mL) | 647.71, (524.66-843.86) | 720.43, (524.56-1803.11) | 0.232 |
| HSP40 (pg/mL) | 1027.08, (878.77-1824.30) | 1177.16, (494.29-1516.41) | 0.452 |
| HSP60 (pg/mL) | 1112.77, (721.15-2220.07) | 1234.40, (808.76-2053.08) | 0.690 |
| HSP70 (pg/mL) | 331.96, (122.30-814.94) | 336.96, (112.32-2650.72) | 0.452 |
| HSP90 (pg/mL) | 568.33, (320.46-2196.38) | 455.22, (134.38-1207.02) | 0.330 |
Also there was no difference between patients in remission after discontinuation of steroid therapy, those in remission but still using steroids, and those with active proteinuria despite steroid treatment in the INS group according to HSPs (P > 0.05).
4. Discussion
In this study, we demonstrated that serum levels of HSPs were significantly higher in patients with IgAN/IgAVN than in the healthy controls, and also than in the patients with INS. This result suggests that HSPs may be involved in pathogenesis and/or progression of IgAN in children. The progression of IgAN is not fully understood although there are many factors that affect disease progression, which lead to glomerular and tubulointerstitial injury (7). Deposition of polymeric IgA can lead to increase of various cytokines and growth factors, for example interleukin 1, 6, tumor necrosis factor, PDGF, TGF - beta, VPF/VEGF in renal cells (7). Local production of these cytokines may increase the mesangial proliferation in children with IgAN (7). Also, excessive oxidative stress may contribute to glomerular injury (7). Cytokines, reactive oxygen species, and proteinuria may lead to tubulointerstitial injury in IgAN (7). All these stress factors and apoptosis lead to renal fibrosis and progression of the disease (7). The same stress factors may contribute to increase of HSPs in our patients because HSPs exhibit a regulatory role in various stress conditions including oxidative, osmotic, ischemic, and toxic injury.
The severity of proteinuria, presence of crescents, and high scores of Oxford classification in renal biopsy specimens were related to poor prognosis in IgAN/IgAVN in children (7). We expected an association between these prognostic factors and HSPs because HSPs are intracellular danger signals. Therefore, we evaluated the relationship between these biomarkers and the presence of proteinuria as a clinical prognostic factor and both presence of crescent and Oxford classification as pathological prognostic factors. HSP27 was the only HSP related to the presence of proteinuria in our study. None of HSPs was related with the other prognostic factors. Podocyte depletion was reported to be related to proteinuria and glomerulosclerosis in IgAN (7, 19). It has been demonstrated that HSP27 can regulate actin cytoskeletal response of podocytes in in vitro model of podocyte injury (20, 21). Increased serum HSP27 levels of the patients with proteinuria in IgAN/IgAVN group may be related to a mechanism of response to the podocyte injury.
HSP27 and 90 were significantly higher in the IgAN/IgAVN whereas they were comparable in the INS and control groups unlike other HSPs, however there are overlaps among the three groups regarding serum levels of these markers. Among our results, HSP60 is the most remarkable biomarker because HSP60 levels in IgAN/IgAVN group versus the other groups which not only were significantly different, but also had no overlap at all as shown in Figure 1. This suggests that HSP60 may be related to the pathogenesis of IgAN/IgAVN. Lang et al. (5) determined that recombinant HSP60 injection aggravated the kidney damage such as glomerular necrosis and tubulointerstitial damage in rat model of immune - mediated glomerulonephritis.
HSP47, as a member of HSP40, is associated with processing and secretion of collagen. This HSP may prevent abnormal collagen secretion (1). Expression of HSP47 in kidney was found to be increased in the patients with IgAN (3). Our results suggest that the serum levels of HSP40 may reflect increases in HSP40 expression in the kidney. Chebotareva et al. (4) demonstrated that urinary excretions of HSP70 were increased especially in patients who had chronic glomerulonephritis. HSP70 is generally considered as a protective chaperone in kidney diseases (1). Maybe some HSPs have protective roles and some have an aggravating role in IgAN/IgAVN. Our study is a clinical study and cannot explain the reason of increases of HSPs in IgAN/IgAVN.
Our study has some limitations. One of these limitations is that our patients were not in the acute phase of the illness. The mean duration between kidney biopsy and blood sampling for the study was 35 months. We believe that further studies in larger groups of patients in the acute phase of the illness may reveal a relationship between these pathologic prognostic factors. The second limitation is our relatively small sample size.
In conclusion, our results demonstrated that serum levels of HSP27, 40, 60, 70, 90 increased in the patients with IgAN/IgAVN considering HSPs might have a role in pathogenesis of the disease. Among these HSPs, HSP60 was a prominent marker because it had no overlap between patients and controls. Explaining the role of these HSPs in IgAN/IgAVN may pave the way for future treatment.
References
-
1.
Beck FX, Neuhofer W, Muller E. Molecular chaperones in the kidney: distribution, putative roles, and regulation. Am J Physiol Renal Physiol. 2000;279(2):F203-15. [PubMed ID: 10919839]. https://doi.org/10.1152/ajprenal.2000.279.2.F203.
-
2.
Sreedharan R, Van Why SK. Heat shock proteins in the kidney. Pediatr Nephrol. 2016;31(10):1561-70. https://doi.org/10.1007/s00467-015-3297-x.
-
3.
Razzaque MS, Kumatori A, Harada T, Taguchi T. Coexpression of collagens and collagen-binding heat shock protein 47 in human diabetic nephropathy and IgA nephropathy. Nephron. 1998;80(4):434-43. [PubMed ID: 9832643]. https://doi.org/10.1159/000045217.
-
4.
Chebotareva NV, Neprintseva NV, Bobkova IN, Kozlovskaia LV. [Investigation of 70-kDa heat shock protein in the serum and urine of patients with chronic glomerulonephritis]. Ter Arkh. 2014;86(6):18-23. [PubMed ID: 25095651].
-
5.
Lang A, Benke D, Eitner F, Engel D, Ehrlich S, Breloer M, et al. Heat shock protein 60 is released in immune-mediated glomerulonephritis and aggravates disease: in vivo evidence for an immunologic danger signal. J Am Soc Nephrol. 2005;16(2):383-91. [PubMed ID: 15601747]. https://doi.org/10.1681/ASN.2004040276.
-
6.
Lebherz-Eichinger D, Krenn CG, Roth GA. Keratin 18 and heat-shock protein in chronic kidney disease. Adv Clin Chem. 2013;62:123-49. [PubMed ID: 24772666].
-
7.
Nakanishi K, Yoshikawa N. Immunoglobulin A Nephropathies in Children (Includes HSP). In: Avner ED, Harmon WE, Niaudet P, Yoshikawa N, Emma F, Goldstein SL, editors. Pediatric Nephrology. 7th ed. Berlin Heidelberg: Springer-Verlag; 2016. p. 983-1033. https://doi.org/10.1007/978-3-662-43596-0_28.
-
8.
Shima Y, Nakanishi K, Hama T, Mukaiyama H, Togawa H, Hashimura Y, et al. Validity of the Oxford classification of IgA nephropathy in children. Pediatr Nephrol. 2012;27(5):783-92. [PubMed ID: 22134880]. https://doi.org/10.1007/s00467-011-2061-0.
-
9.
Coppo R, Davin JC. The difficulty in considering modifiable pathology risk factors in children with IgA nephropathy: crescents and timing of renal biopsy. Pediatr Nephrol. 2015;30(2):189-92. [PubMed ID: 25318618]. https://doi.org/10.1007/s00467-014-2954-9.
-
10.
Ozen S, Duzova A. Henoch-Schonlein Purpura, Polyarteritis Nodosa, Wegener's Granulomatosis, and Other Vasculitides. In: Cimaz R LT, editor. Pediatrics in Systemic Autoimmune Diseases. 1st ed. Amsterdam: Elsevier; 2014. p. 151-67.
-
11.
Kang Y, Park JS, Ha YJ, Kang MI, Park HJ, Lee SW, et al. Differences in clinical manifestations and outcomes between adult and child patients with Henoch-Schonlein purpura. J Korean Med Sci. 2014;29(2):198-203. [PubMed ID: 24550645]. [PubMed Central ID: PMC3923997]. https://doi.org/10.3346/jkms.2014.29.2.198.
-
12.
Dillon MJ, Ozen S. A new international classification of childhood vasculitis. Pediatr Nephrol. 2006;21(9):1219-22. [PubMed ID: 16821024]. https://doi.org/10.1007/s00467-006-0181-8.
-
13.
Working Group of the International Ig AN, the Renal Pathology S, Roberts IS, Cook HT, Troyanov S, Alpers CE, et al. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int. 2009;76(5):546-56. [PubMed ID: 19571790]. https://doi.org/10.1038/ki.2009.168.
-
14.
Roberts IS. Pathology of IgA nephropathy. Nat Rev Nephrol. 2014;10(8):445-54. [PubMed ID: 24861083]. https://doi.org/10.1038/nrneph.2014.92.
-
15.
A report of the International Study of Kidney Disease in Children. The primary nephrotic syndrome in children. Identification of patients with minimal change nephrotic syndrome from initial response to prednisone. J Pediatr. 1981;98(4):561-4. [PubMed ID: 7205481].
-
16.
National High Blood Pressure Education Program Working Group on High Blood Pressure in C; Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 Suppl 4th Report):555-76. [PubMed ID: 15286277].
-
17.
Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20(3):629-37. [PubMed ID: 19158356]. [PubMed Central ID: PMC2653687]. https://doi.org/10.1681/ASN.2008030287.
-
18.
Yap HK, Lau PY. Hematuria and Proteinuria. In: Geary FD SF, editor. Comprehensive Pediatric Nephrology. 1st ed. Philadelphia: Mosby Elsevier; 2009. p. 179-93.
-
19.
Xu L, Yang HC, Hao CM, Lin ST, Gu Y, Ma J. Podocyte number predicts progression of proteinuria in IgA nephropathy. Mod Pathol. 2010;23(9):1241-50. [PubMed ID: 20495534]. https://doi.org/10.1038/modpathol.2010.110.
-
20.
Smoyer WE, Gupta A, Mundel P, Ballew JD, Welsh MJ. Altered expression of glomerular heat shock protein 27 in experimental nephrotic syndrome. J Clin Invest. 1996;97(12):2697-704. [PubMed ID: 8675679]. [PubMed Central ID: PMC507361]. https://doi.org/10.1172/JCI118723.
-
21.
Smoyer WE, Ransom RF. Hsp27 regulates podocyte cytoskeletal changes in an in vitro model of podocyte process retraction. FASEB J. 2002;16(3):315-26. [PubMed ID: 11874981]. https://doi.org/10.1096/fj.01-0681com.