1. Background
The inflammatory bowel diseases (IBD), including ulcerative colitis (UC), Crohn’s disease (CD), and IBD unclassified (IBDU), comprise the most important group of chronic gastrointestinal disorders (1). It is estimated that up to 25% of patients with IBD are diagnosed in childhood and adolescence (1, 2). Recent data demonstrates increasing rates of IBD, especially in Eastern countries (3, 4).
The most common features of IBD are intermittent gastrointestinal symptoms such as diarrhea and abdominal pain. Children with IBD may also be impacted by weight loss, poor weight gain, impaired linear growth and delayed pubertal development. Additionally, extra-intestinal manifestations such as fatigue, anorexia, joint, and skin symptoms, are commonly seen (5).
IBD can adversely affect children in many different ways, with psychosocial and educational impacts such as interruptions to schooling and interference with normal sporting pursuits. Taken together, these various adverse effects can impact the quality of life (QOL) of these children. Health-related quality of life (HRQOL) involves all the different aspects of life quality, physical and mental perceptions of health that could exert an overall effect on an individual’s health (4, 6). HRQOL can be measured by various generic instruments that focus on physical, emotional, social, and cognitive dimensions (5, 7-10).
To meet the need for a valid disease-specific HRQOL assessment in children with IBD, the IMPACT questionnaire was designed (5).The current version, IMPACT-III, is now the preferred questionnaire for evaluation of HRQOL in children with IBD and has been cross-culturally adapted and revalidated in various countries and settings (11-16).
2. Objectives
The aim of this study was to establish the validity and reliability of a Farsi/Persian version of the IMPACT-III tool in Iranian children with IBD testing the factor structure, internal reliability and synchronized validity. Additionally, we aimed to compare the factor scores with the Croatian, British, and Swiss versions.
3. Methods
Patient Background Characteristics: Children aged between 10 - 17 years old diagnosed with IBD for at least six months seen in clinics at Imam Khomeini Hospital, Tehran, Iran and Ghaem Hospital, Mashhad, Iran from June 2013 to Jan 2014 were recruited. After obtaining informed consent from the children and their parents, each participant completed the questionnaires in a face to face interview. Key background demographic data (including gender, age, socioeconomic status and disease classification) were collected and an assessment of current IBD disease activity was also performed.
We included every child aged between 10 - 17 years old diagnosed with IBD for at least six months, the only exclusion criterion was the refusal by the child or parent to be involved in the study.
The current activity of UC is graded according to the Montreal classification: S0, (recovery) no signs or symptoms; S1, (mild) four or fewer stools per day (with or without bleeding) and lack of systemic symptoms with normal inflammatory markers; S2, (moderate) four stools a day with mild systemic symptoms; S3, (severe) six times or more stools per day, pulse rate greater or equal to 90 beats per minute, temperature greater than 37.5° Celsius, hemoglobin concentration less than 10.5 g/L, ESR equal or greater than 30 mm/h. The socioeconomic status of each child’s family was measured based on a questionnaire designed by the researchers (available on request) and includes the level of education, household monthly income, and the amount of bank savings.
The IMPACT-III Questionnaire: The IMPACT-III questionnaire has 35 items regarding the frequency and intensity of the effects of IBD on different aspects of health in the last two weeks by using a 5-point Likert response scale (11). The IMPACT-III consists of 6 subscales which are computed by summing the responses for each question within a domain: bowel symptoms (7 items), systemic symptoms (3 items), emotional functioning (7 items), social functioning (12 items), body image (3 items), and treatment/interventions (3 items). Higher IMPACT-III scores indicate better HRQOL. To measure the overall HRQOL, a total score was calculated by summing all 35 items.
Translation of the IMPACT-III Into Farsi: Translation of the IMPACT-III into Farsi involved a classic “forward-backward” translation method (17). First of all, the original questionnaire in English was translated into Farsi by two bilingual translators; one of them had medical knowledge, and was familiar with the concept of health-related quality of life. Then, a committee that included the two translators, a methodologist, a clinician, and a language specialist synthesized and reviewed the two obtained translations. Third, two different translators with the source language (English) as their mother tongue, blinded to the original version, translated the instrument into English again. Eventually, the expert committee reviewed and synthesized all translations for the second time to elaborate Farsi items similar to the original English items and to ensure readability.
Cognitive Debriefing and Finalization: In this step children with Crohn’s disease or UC were selected to complete the questionnaire. Then, an interviewer who was bilingual in both English and Farsi, familiar with IMPACT-III and cognitive debriefing process, reviewed each question with the participant alternative wording, understanding, interpretation, and cultural relevance of the translation. Following that, the results and patients’ interpretations were compared with the original version to amend disparities. Finally, any possible typographic, grammatical, or spelling errors were corrected in the proofreading process and the final report was sent to the IMPACT-III developer to certify the Iranian/Farsi IMPACT-III version.
Testing the Farsi Version of the IMPACT-III Questionnaire: A convenience sample of children tested the relevance, clarity, and fluency of the obtained questionnaire.
Statistical Analysis: The total score of the IMPACT-III was the sum of the scores for each item. After gathering all required data, statistical analyses were undertaken with SPSS 16 (SPSS Inc. Released 2007. SPSS for Windows, version 16.0. Chicago, US).
To test the factor structure of the questionnaire, principle component analysis was done. After that, exploratory factor analysis was performed according to the Kaiser criteria (18). Principal components analysis was performed individually on each question of the questionnaire and the factor loading was assessed for each item. The final principal component analysis presented a four scale structure. Each item with a factor loading > 0.4 entered the scales. Spearman correlation coefficient was used to assess the association between the IMPACT-III total score and clinical variables. Factor analysis was done to determine optimum domain structure, use of Cronbach’s alpha coefficients to test internal reliability, ANOVA to assess discriminate validity and use of intra-class correlation coefficients to assess test-retest reliability. Discriminate validity assessment was done with Mann-Whitney U test for comparing factors between the active disease and inactive disease groups. t-test and chi-square test were used to compare quantitative and qualitative data between the two groups of patients with active and inactive diseases. A P value of less than 0.05 was considered significant.
Ethical Consideration: The study was approved by the ethics committee of Mashhad University of Medical Sciences, Iran. All patient related information was kept confidential and deleted after data analysis.
4. Results
Patients: Eighty-nine children were recruited to contribute to the assessment of the Farsi version of the IMPACT-III questionnaire. The background demographics and characteristics of all patients were collated (Table 1).
| Values | |
|---|---|
| Age, y | |
| 11 - 19 | 14.2 ± 2.1 |
| Sex | |
| Male | 48 (54) |
| Female | 41 (46) |
| Disease | |
| Crohn’s disease | 21 (24) |
| Ulcerative colitis | 68 (76) |
| Disease activity, % | |
| Active | 46 |
| Inactive | 54 |
| Residency | |
| Urban | 56 (63) |
| Rural | 33 (37) |
aValues are expressed as mean ± SD or No. (%).
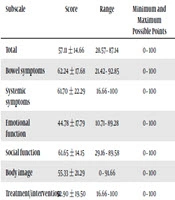
QOL Scores of Patient Groups: Children with active disease had lower scores than those with inactive disease (65.3 ± 12.2 versus 47.5 ± 11.1; P < 0.001) (Table 2). In addition the children with UC had higher IMPACT-III scores than the children with CD (P = 0.001). IMPACT-III scores did not differ according to gender, rural or urban residential status, age or socio-economic status (P > 0.05 for all). Score of different scales of the questionnaire and scores of all participants in the 6 subscales of the questionnaires are summarized in Tables 3 and 4.
| Disease Activity | Number | Mean | Std Deviation | Std Error Mean |
|---|---|---|---|---|
| Total score | ||||
| Inactive | 48 | 65.3 | 12.2 | 1.8 |
| Active | 41 | 47.5 | 11.1 | 1.7 |
| Subscale | Score | Range | Minimum and Maximum Possible Points |
|---|---|---|---|
| Total | 57.11 ± 14.66 | 28.57 - 87.14 | 0 - 100 |
| Bowel symptoms | 62.24 ± 17.68 | 21.42 - 92.85 | 0 - 100 |
| Systemic symptoms | 61.70 ± 22.29 | 16.66 - 100 | 0 - 100 |
| Emotional function | 44.78 ± 17.79 | 10.71 - 89.28 | 0 - 100 |
| Social function | 61.65 ± 14.15 | 29.16 - 89.58 | 0 - 100 |
| Body image | 55.33 ± 21.29 | 0 - 91.66 | 0 - 100 |
| Treatment/intervention | 52.90 ± 19.50 | 16.66 - 100 | 0 - 100 |
aValues are expressed as mean ± SD.
| Bowel Symptomsb | Emotional Function | Systemic Symptomsb | Social Function | Body Imageb | Treatment/Intervention | |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Male | 62.20 ± 17.83 | 47.02 ± 15.82 | 64.23 ± 21.19 | 63.28 ± 13.51 | 53.64 ± 21.80 | 53.64 ± 17.86 |
| Female | 62.28 ± 17.73 | 42.16 ± 19.72 | 58.74 ± 23.41 | 59.75 ± 14.79 | 57.31 ± 20.76 | 52.03 ± 21.47 |
| P value | 0.97 | 0.2 | 0.33 | 0.24 | 0.34 | 0.70 |
| Intensity | ||||||
| Severe | 38.56 ± 10.43 | 26.26 ± 7.57 | 38.72 ± 16.38 | 46.32 ± 6.61 | 47.06 ± 13.48 | 32.84 ± 18.51 |
| Nonsever | 67.80 ± 14.09 | 49.15 ± 16.67 | 67.12 ± 19.97 | 65.27 ± 12.98 | 57.29 ± 22.37 | 57.63 ± 16.59 |
| P value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | 0.04 | < 0.001 |
| Type | ||||||
| Crohn | 53.23 ± 17.23 | 35.03 ± 15.87 | 51.58 ± 24.09 | 54.26 ± 13.21 | 46.42 ± 19.99 | 41.66 ± 20.41 |
| UC | 65.02 ± 16.99 | 47.79 ± 17.36 | 64.82 ± 20.90 | 63.93 ± 13.72 | 58.08 ± 21.06 | 56.37 ± 17.99 |
| P value | 0.006 | 0.003 | 0.02 | 0.005 | 0.019 | 0.002 |
| Residency | ||||||
| Urban | 63.64 ± 17.74 | 46.93 ± 18.75 | 64.43 ± 21.23 | 63.05 ± 14.30 | 59.22 ± 21.12 | 54.61 ± 20.47 |
| Rural | 59.84 ± 17.58 | 41.12 ± 15.62 | 57.07 ± 23.58 | 59.28 ± 13.78 | 48.73 ± 20.21 | 50 ± 17.68 |
| P value | 0.33 | 0.068 | 0.18 | 0.2 | 0.015 | 0.28 |
| Economic | ||||||
| 0 | 64.75 ± 13.46 | 45.03 ± 16.50 | 60.86 ± 21.96 | 63.67 ± 11.93 | 50.72 ± 23.82 | 55.43 ± 18.73 |
| 1 | 66.57 ± 17.88 | 51.28 ± 19.93 | 67 ± 18.39 | 62 ± 14.26 | 63.66 ± 19.37 | 60.33 ± 20.16 |
| 2 | 57.5 ± 20.75 | 40.35 ± 16.91 | 60.41 ± 24.46 | 59.16 ± 16.98 | 53.75 ± 19.20 | 47.5 ± 19.13 |
| 3 | 61.26 ± 15.62 | 45.05 ± 17.40 | 65.38 ± 23.77 | 66.34 ± 14.72 | 59.61 ± 17.95 | 51.28 ± 19.19 |
| 4 | 54.91 ± 21.50 | 34.37 ± 11.60 | 44.79 ± 22.68 | 53.38 ± 8.10 | 39.58 ± 20.77 | 38.54 ± 10.85 |
| P value | 0.36 | 0.1 | 0.14 | 0.26 | 0.046 | 0.036 |
aValues are expressed as mean ± SD.
bNon-parametric tests were used as data was not normally distributed; P value less than 0.05 (shown in bold) indicates the scope of the relevant differences between subgroups were statistically significant.
The Reliability of the Questionnaire: Alpha coefficients of all the scales were convincing except the scale of treatment/intervention (Cronbach α = 0.9).
4.1. Principal Components Analysis to Examine the Scope of the Questionnaire for Iranian Respondents
4.1.1. Primary Analysis
Sufficiency of the Cases in the Study: Kaiser-Meyer-Olkin (KMO) index for this study was 0.80, which indicated that the number of case studies to perform principal component analysis (PCA) was sufficient.
Sphericity (Correlation of the Questionnaire Questions): Ratio chi-square of Bartlett test to check sphericity was calculated at 2064. PCA performing was possible with respect to df = 595 and P-value < 0.001, which indicated a significant correlation between the items of the questionnaire.
4.1.2. Confirmatory Factor Analysis
The loading coefficient of each question in the 6 scopes requested in factor analysis is shown in the Appendix 1 in Supplementary File).
The sextuplet categorization covers 63.23% of total variance. According to the distribution of questions in different subscales, three of subscales (bowel symptoms, systemic symptoms, and body image) showed a sufficient distribution, but for other subscales there was no sufficient distribution.
4.1.3. Exploratory Factor Analysis
After performing the factor analysis, based on Kaiser Criteria the extracted scales, had Eigen values higher than 1. Analysis of this study data, suggested 10 scales, which covers 75.54% of the total variance. Six of the 10 suggested scales had the maximum of one or two items and showed low internal reliability (Cronbach’s alpha coefficient was calculated almost 0.24 for the third scale). As a result, it does not seem to fit the scope composition of the questionnaire.
Five Subscales Solution: The default domain of the five-factor analysis was conducted on the questionnaire. The subscales could cover a total of 55.6% of the total variance. One area of the sphere scaling, covered only one item and in other scales, internal reliability was lower than 0.32. Therefore, the combination of the scope of the questionnaire does not seem right.
Four Subscales Solution: The subscales could cover 72% of the total variance. Internal reliability of these scales is acceptable. The current data demonstrated Cronbach’s alpha coefficient of 0.90 for physical functioning, 0.85 for emotional functioning, 0.82 for social functioning, and 0.61 for body image.
4.1.4. Discriminative Validity
At this stage, potential new areas in the questionnaire were examined to distinguish the group of patients with active disease from those with inactive disease (Table 5). Based on the responses of these Iranian children, the new mining areas in the questionnaire could distinguish between the groups of patients with severe and mild-moderate illness.
| New Mined Areas | Factors Number | Total Score | The Inactive Disease Group Score | The Active Disease Group Score | P Value |
|---|---|---|---|---|---|
| 1 | 14 | 55.33 ± 1.92 | 60.88 ± 1.76 | 31.82 ± 2.18 | < 0.001 |
| 2 | 8 | 60.39 ± 1.66 | 63.02 ± 1.83 | 49.26 ± 2.69 | < 0.001 |
| 3 | 5 | 63.48 ± 1.94 | 67.90 ± 1.98 | 44.74 ± 2.74 | < 0.001 |
| 4 | 4 | 38.55 ± 1.76 | 42.27 ± 1.87 | 22.79 ± 2.20 | < 0.001 |
aValues are expressed as mean ± SD.
bComparisons were made used Mann-Whitney testing, with P < 0.05 being significant.
5. Discussion
This is the first study to translate and validate a Farsi version of the IMPACT-III questionnaire. In Iranian children suffering from IBD, the IMPACT-III questionnaire illustrated that QOL was associated with disease activity but did not differ according to gender or geography. The current data also showed that the original six domain scales were not used among Iranian children. This result is supported by two other studies testing the factor structure of the IMPACT-III (19, 20). Moreover, these analyses showed low internal reliability in the original scales and treatment/interventions.
In this study, a PCA with the extraction of four items showed the most useful and appropriate solution. This scaling is in line with Werner et al. (15). Surprisingly, the sub-scaling in the current study was also similar to that described by Werner et al.: these subscales were well-being/feelings, emotional symptoms, social behavior and body image.
A four-factor scaling method has been suggested by Perrin et al. (21) in their study that assessed the factor structure of the English version of IMPACT-II questionnaire (an earlier version of the IMPACT-III) among 220 American participants aged between 8 and 18 years of age. This version included scales, which described the same HRQOL dimensions as evaluated in the current study (well-being/symptoms, emotional functioning, social interactions, and body image). The main difference between the four-factor scales of the US, Swiss, and the Iranian versions was the number of items per scale. This might be due to the different factor thresholds for inclusion.
Further, the choice of cut-offs differs in different studies. Perrin et al. (21), included items with factor loadings greater than 0.3 in a factor while Werner et al. (15) included factor loadings greater than 0.5. Factor loadings greater than 0.4 were included in the current study. In comparison to the Swiss version, the US and the current studies have more items in each scale.
Two other available studies which were conducted in England and Croatia, suggested a five-factor solution (13, 14). This was less suitable than the four factor analysis in Iranian children. An exploratory factor analysis in Iranian children showed that a five-factor solution had a lower internal reliability (about 0.32) than the four-factor solution. The current data demonstrated Cronbach’s alpha coefficient of 0.90 for physical functioning, 0.85 for emotional functioning, 0.82 for social functioning, and 0.61 for body image.
Furthermore, the four-scaling solution contains a good discriminate validity for four domains (gastrointestinal symptoms, social functioning, emotional functioning, and body image). Participants, who were in the active phase of the disease, had lower social and emotional functions and more negative body image than those in their inactive phase of the disease (P < 0.001).
5.1. Study Limitations
First of all, the advantages of this study were large sample size, high response rate, and the use of standardized and well-validated instruments for the assessment of IBD activity and HRQOL. However, a limitation of this study was that the subjects were recruited from just two referral hospitals in Iran. Consequently, this population might be biased towards children with more severe or uncontrolled disease and might not be representative of all children with IBD in Iran. In addition, this study did not compare the IMPACT-III responses to other generic measurements of QOL.
5.2. Conclusions
This study indicates convincing validity and reliability for the four-factor IMPACT-III scores regarding HRQOL in Iranian children with IBD. The Farsi version of this questionnaire can now be used in clinical practice in children with IBD in Iran. The factor structure of the questionnaire should be assessed in further studies with larger sample size.