1. Background
The incidence of empyema is growing all over the world and causes childhood morbidity, developing in about 0.6% of childhood pneumonias (1). Pleural effusion during nonspecific bacterial pneumonia, malnutrition, immunodeficiency, and irregular antibiotic therapy becomes empyema for a variety of reasons including contamination during thoracentesis, delayed diagnosis of pneumonia, the tendency to use antibiotics in acute phase in pediatric clinics, and disappearance of the signs and symptoms of pneumonia (2). Besides antibiotics or thoracentesis, there are a variety of treatment options for empyema treatment including chest drain placement, mini-thoracotomy, chest drainage and fibrinolytic, open decortication and VATS, available (3).
Yet, standards of treatment are not formed, and local practice and choice of medical doctor still determine the method of patient care. These treatment regimens and success rates may vary in relation to the stage of empyema (3). In various controlled researches, it was presented that intrapleural fibrinolytic agents (urokinase and streptokinase) were safe. Fibrinolysis is used in some centers and in case of failure, the open decortication or VATS are applied (4, 5).
2. Objectives
This research aimed to evaluate different treatment alternatives in the PPE therapy in pediatric age group.
3. Methods
3.1. Study Group
This study included 681 (379 males and 302 females) pediatric patients with an average age of 4.8 years (ranging from 30 days to 15 years) with PPE between 2000 and 2016 at two separate centers (Sakarya University Medical Faculty and Yüzüncüyıl University Medical Faculty), Turkey. Both centers are connected to the same institution. The sociocultural and economic levels of the people living here are approximately the same, so there was no difference between the centers. Also the principle of approach to patients was the same. Our working principle has not changed over the years. However, the number of patients gradually decreased. We think this is due to the increased use of antibiotics. In addition, VATS debridement method has been applied more in recent years.
Patients were included in the study if they were under 15 years old and if the radiographic findings (chest X-ray, ultrasonography or pleural fluid on computed tomography) of the empyema were present.
Hemoglobin, clotting profile, blood culture, platelet count, white blood cell (WBC) and differential counts, electrolytes, C-reactive protein (CRP), lactate dehydrogenase (LDH), and albumin were estimated in each patient. Chest ultrasonography or computed tomography was used to choose the best location for thoracentesis to reach pleural effusions and to separate pleural from an intraparenchymal process.
Following criteria were used to diagnose empyema: (1) pleural fluid glucose level below 50 mg/dL, (2) gross purulent pleural fluid documented by thoracentesis, (3) pleural fluid pH level below 7.00, (4) positive gram stain, (5) liquid lactic dehydrogenase level > 1000 IU/L.
The treatment modality was applied step-by-step through simple procedure to invasive processing.
Firstly, using Student t-test, characteristics of the six groups were compared. The analyses of all data were performed considering treatment principles. A value of P < 0.05 was regarded as statistically significant. The patients were periodically controlled at the policlinic on the 7th, 21st and 60th day after discharge. The average long-term follow-up was between 6 and 18 months.
3.2. Treatment Groups
Patients were classified in six groups according to the treatment applied: group A (n = 34): thoracentesis alone and broad-spectrum antibiotic therapy, group B (n = 298): chest tube drainage alone, group C (n = 81): chest tube drainage and intrapleural fibrinolytic therapy, group D (n = 141): chest tube drainage with video-assisted thoracoscopic surgery, group E (n = 110): primary operation following chest tube drainage, group F (n = 17): primary operative management without chest tube drainage.
Thoracentesis and wide-spectrum antibiotics treatment: firstly, more sensitive drugs and broad-spectrum antibiotics were given to all patients for the treatment. Initial treatment for acute empyema and parapneumonic effusions is pleural aspiration plus lavage, by which 34 (4.9%) patients were treated.
3.3. Chest Tube Treatment
Chest tube treatment was the second step. Intrapleural fibrinolytic therapy or decortication was considered in patients with clinically inadequate iv antibiotics and drainage of closed tube thoracostomy. CT scan and/or thoracic ultrasonography and/or CT scan were performed to determine if loculations were present and if yes where they are located in patients with insufficient drainage. A second chest tube was placed in these patients when necessary and adequate drainage was provided.
3.4. Fibrinolytic Treatment
Streptokinase or urokinase as 250.000 or 100.000 U, respectively, in 100 mL of 0.9% saline solution was administered daily in the chest tube. It was held for four hours. The tube was then opened and placed under 10 - 20 cm H2O negative suction pressure until the next application. The patient was encouraged to mobilize. This treatment lasted for 2 - 10 days assessing daily chest radiographs or chest tomography. Hematocrit levels and clotting parameters were tracked regularly. Patients were also monitored considering evidence of respiratory decompensation, bleeding, chest pain and anaphylaxis. Failure was defined as the persistence of pleural fluid in thoracic ultrasonography and persistent fever four days after intervention. VATS or decortication was performed in those who failed to respond to fibrinolytic therapy.
3.5. VATS
Taking into account the size of the child, VATS was administered under general anesthesia with one or two lung ventilations. Free fluid and loculations were evacuated, fibrinous adhesions removed, pleural fibrin was extracted from the pleural lining using an endoscopic holding forceps and aspirated. One or two chest drains were located after the procedure. To facilitate further drainage postoperatively, the drains were attached to the negative suction pressure of 10 - 20 cm H2O. Achieving a little drainage (30 - 50 mL/24 hours), chest drains were removed, and the patient discharged.
3.6. Primary Operation (Decortication) with Chest Tube Treatment
Treatment with decortication was planned for patients all of whom were not healed clinically by the application of intrapleural fibrinolytic therapy or iv antibiotics and closed tube thoracostomy drainage. Decortication was also performed in patients with obvious pulmonary trapping. All of the decortications were conducted with posterolateral thoracotomy. The pleural cavity was entered via the fifth intercostal space. The fibrinous shell on the surface of visceral and parietal pleura was removed entirely.
3.7. Primary Operation Without Chest Tube Drainage
Direct decortication was performed in patients with pulmonary trapping who were judged unable to benefit from drainage. In those with negative or minimal initial thoracentesis on admission, primary decortication was carried out without trying chest tube drainage if radiological investigation revealed solidified pleural material and significant compression of the lung.
4. Results
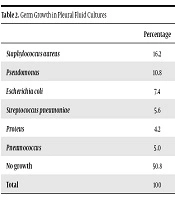
The results are presented in Table 1 in a summarized form. Results from pleural fluid cultures are presented in Table 2. The most common causative microorganism was Staphylococcus aureus [16.1% (n = 110)]. Pleural fluid culture, however, was negative in 345 (50.6%) cases. In our study, no tuberculosis bacilli growth was observed in pleural fluid cultures. However, tuberculous empyema was detected in 12 patients who underwent decortication and it was confirmed in pathology reports. Antituberculosis treatment was started in these patients. Radiographic septations were seen in most of the patients except group A who were treated with thoracentesis and antibiotherapy. There was no correlation between culture positivity and septation.
| Groups | No. (%) |
|---|---|
| Group A | 34/34 (100) |
| Group B | 298/630 (47) |
| Group C | 67/81 (82.7) |
| Group D | 141/141 (100) |
| Group E | 110/110 (100) |
| Group F | 17/17 (100) |
| Percentage | |
|---|---|
| Staphylococcus aureus | 16.2 |
| Pseudomonas | 10.8 |
| Escherichia coli | 7.4 |
| Streptococcus pneumoniae | 5.6 |
| Proteus | 4.2 |
| Pneumococcus | 5.0 |
| No growth | 50.8 |
| Total | 100 |
Appropriate intravenous antibiotics and sequences of thoracentesis were used in the treatment of group A. Group B patients were similarly treated with proper antibiotics and tube thoracostomy and discharged. For intrapleural fibrinolytic therapy, streptokinase (51 patients) and urokinase (30 patients) were administered with chest tube drainage.
Length of hospitalization was 19.5 (range 5 - 27) days after application of an average dose of fibrinolytic. After instillation of the fibrinolytic agent in all patients, it was observed that chest tube drainage increased, loculations resolved radiographically, and the cavity shrunk. Most of the drainage occurred in the first 48 hours. Clotting parameters of all our patients before and after fibrinolytic treatment were within reasonable limits. In 67 patients to whom fibrinolytic therapy was applied, a complete or partial response was obtained. Treatment was not effective in 14 of 81 patients receiving urokinase or streptokinase instillation. Thus thoracotomy or VATS was performed. Complete healing was observed after the application of thoracotomy or VATS. One patient died due to an allergic reaction and pleural bleeding.
Decortication was performed in 140 patients (due to the trapped lung in chest tomography) after the chest tube drainage. Seventeen patients underwent decortication as the first treatment after radiological examination without application of closed tube thoracostomy. Pulmonary resection was performed in 15 patients in addition to decortication. Lobectomy, bilobectomy, wedge resection, and segmentectomy were performed in 3, 1, 7, and 4 patients in addition to decortication. All the patients in the thoracotomy group healed entirely. No deaths occurred (Table 2).
Nine (1.32%) patients died due to pneumonia, sepsis, pleural bleeding and heart failure. Congestive heart failure and pneumonia were seen in four of these patients in the first arrival to the hospital. Four children died due to sepsis. Treatment with intrapleural streptokinase was quitted due to hemorrhagic drainage in a patient with fibrinopurulent phase empyema. This patient died two days later because of a septic condition.
All the patients in the thoracotomy group recovered entirely. No deaths occurred. Postoperative complications (23.7%) as atelectasis, incisional infection, delayed expansion, and reoperation were observed in 40, 22, 17 and 2 patients. Wound infection was treated with antibiotics according to the culture result (Pseudomonas aeruginosa). Atelectasis was treated by breathing exercises and nasotracheal aspiration, and therefore no bronchoscopy was required. A 300-mL-per-day chest tube blood leak in a 7-year-old was a re-operation indication. Intercostal artery ligation was performed. Postoperative length of hospitalization was averagely 9 (6 - 18) days.
Among the six treatment groups, there was a clinically significant difference in hospital stay after the intervention.
According to the duration of chest tube, the difference of group B and group E, and that of group C and group E was statistically significant, in favor of group E (P < 0.000). According to the length of hospital stay, its difference between groups A and B, A and C, and A and D was significant in favor of group A (P < 0.001, P < 0.006, and P < 0.000, respectively). Also, the difference between group E and group F was significant in favor of group F (P < 0.001).
The length of hospital stay after treatment was 18.0 in patients treated with VATS compared with 19.5 days in children who received fibrinolytic agents (Table 3).
| Group | A | B | C | D | E | F |
|---|---|---|---|---|---|---|
| Age | 4.5 | 5.2 | 5.1 | 5.4 | 4.7 | 4.2 |
| Duration of initial chest tube (day) | 15.1 | 15.5 | 16.2 | 11.6 | ||
| Post-intervention hospital stay (day) | 8.3 | 19.0 | 19.5 | 18.0 | 21.5 | 9.1 |
| Death | 8 | 1 |
5. Discussion
Various reasons such as pleural effusion, malnutrition, immune deficiency, irregular antibiotic therapy, delayed diagnosis of pneumonia, contamination during thoracentesis, and disappearance of the signs and symptoms of pneumonia cause nonspecific bacterial pneumonia to develop to empyema (1). The treatment time of a patient with empyema in the hospital is longer than that of a parapneumonic effusion. Decortication is the preferred method of treatment if untreated empyema quickly acquires a fibrinopurulent feature and produces more fibrin deposits in the pleural region. Aspiration, if the treatments delayed, often fails in empyema treatment (6) as it was confirmed in this study. Most of the patients did not receive pneumonia treatment in the period prior to being brought to our clinic, and if they did, it was unsatisfying. Therefore, most patients had pleural effusion with viscous pus. As a conclusion, the success rate of aspiration treatment is extremely low. It is sometimes difficult to obtain pleural effusion with thoracentesis even with a large needle.
This study shows a significant clinical difference regarding post-intervention length of hospitalization between chest tube drainage, percutaneous chest drains and intrapleural fibrinolysis and primary VATS in empyema treatment of children. While there are many case series comparing surgical interventions with fibrinolytic treatment, none of them are controlled randomized studies. The goal of giving fibrinolytic agents to the pleural space is to improve drainage by clearing the fibrin filaments and lymphatic pores (7, 8). As the success rate of tube thoracostomy for local empyema is low, alternative approaches have been developed.
Many studies have documented successful drainage of multi-loculated empyema using streptokinase and urokinase administered via a single chest tube (9, 10). Intra-pleural fibrinolysis applied with alteplase, besides the administration of streptokinase and urokinase, has been found to safely enhance pleural drainage and reduce the volume of pleural inflammatory debris (11). However, this study was performed only in a localized clinic. The success rate of fibrinolytic agents administration with open thoracotomy and decortication in multi-loculated cases was 90% in fibrinolytics, but 100% in decortication. In a similar study, two and five patients with urokinase instillation for empyema drainage underwent incomplete drainage of sepsis and decortication, respectively (three recovered, and two died after surgery). More than 45% of patients had to have more than one drain. The average length of hospitalization was 20 days (12). In this research, one (1.2%) child died from hemorrhage in the fibrinolytic group. The partial response rate was 9.8%. The post-intervention average length of hospitalization was 19.5 days. There was no death observed among the patients who were decorticated after fibrinolytic instillation.
VATS rates for primary treatment of empyema in children have gained popularity during the last 15 years. The researchers asserting the application of VATS argue that it has a potential advantage when compared to open surgery to limit morbidity on the skin, nerves, muscle and backbone structures that arise after a major surgical incision (13). However, this causes infection, pain, cosmetic scarring and limitation of movement. Also, cytokine responses may be reduced by VATS as compared to what conventional surgery does (14). But these statements are based on clinical experience rather than carefully conducted studies. The fact that it is heavily based on the surgeon’s skill is the most considerable limitation of VATS. In some centers, researchers have reported poor results (15) because surgical expertise to apply pediatric VATS is confined to several well-equipped centers (16, 17). Angelillo Mackinlay et al. compared 31 patients with 33 patients treated with thoracotomy in the VATS-treated fibrinopurine phase (18). They noted that success rates of VATS treatment and open thoracotomy are the same, yet VATS treatment offers significant advantages when compared to thoracotomy considering solving the disease, length of hospitalization, and cosmetic results.
VATS has been implemented in our clinic for years. The positive results were achieved for most of the cases encountered in this clinic. It is recommended by this study primarily in the fibrinopurine phase. This work was not designed as an equivalent study because hospital stay in this study is longer than that reported in previous studies. Regarding the essential features, six treatment groups in the study were well matched. The length of hospitalization after the intervention for the six groups was detected to be different.
A literature search of randomized and retrospective studies that pinpoint methods of evaluation and treatment of PPE was carried out in Medline and Scopus databases. Small uncomplicated effusions resolve with antibiotics alone, larger ones require small-bore chest tube drainage and in case of complicated loculated PPE, fibrinolysis or VATS should be considered. Both methods promote faster drainage, reduce hospital stay and obviate the need for further interventions when used as first-line approach. However, primary treatment with VATS is not advised by the majority of studies as a first choice intervention, unless medical treatment has failed (19).
The average length of hospital stay before decortication, including other treatment time, was 11.6 days. It was 9.9 days after decortication. The total length of hospital stay was 21.5 days for whom decortication was applied. Compared to other studies the average length of hospital stay in this study was longer. This may be correlated with the requirements of longer preparation and observation times and a relatively high number of multi-loculated chronic cases.
Sonnappa et al. suggested that in the case of examination of the pleural space and the application of thoracoscopic decortication if the surgeon considers VATS in appropriate (prevention of lung enlargement because of thick cortex), the procedure should be converted into mini-thoracotomy. This means that VATS has failed (20). There are no healing or therapeutic benefits considering fibrinolysis and VATS in empyema therapy, whereas VATS is causing a considerable amount of cost. Fibrinolysis may reduce the risk of acute clinical deterioration and in some children with empyema it should be the first-line treatment (17, 21).
Fibrinolytic use is recommended in potential decortication individuals (22). However, surgical morbidity is low, and the mortality rate is infrequent. Unlike studies in agreement with intrapleural fibrinolytic therapy, streptokinase and urokinase in an experimental animal model have not been found to be valid for liquefying thick pleural fluids (23, 24).
Demirhan et al. suggest that chest tube drainage is an effective and safe primary treatment for postpneumonic pediatric empyema. In cases that it is not satisfactory, decortication can be successfully performed with thoracotomy resulting in low mortality and morbidity (25).
Only one small randomized study (26) comparing drainage methods with surgical methods directly achieved an advantage of significant treatment success in the surgical treatment group (44% versus 91%). However, promoting surgical procedures as the first-line treatment will be a disadvantage with the use of more invasive methods in many patients who will be healed with only simple drainage.
The presence of the trapped lung and a thick cortex is an indication for surgery and decortication (3, 22). The removal of fibrinous debris through chest tube also necessitates decortication. If drainage is not practical, decortication should be conducted as soon as possible. The tube may be the initial treatment instead of spending time for thoracostomy. All the patients in group F have been surgically intervened. This approach is recommended in this study when the condition of the patient is appropriate for surgery because of reduced mortality and morbidity, reduced length of hospital stay and quick discharge of the patient. In clinical experience of the researchers in this study, the mortality of decortication is extremely low. This finding supports the view that mortality is superiority in early and limited thoracotomies.
5.1. Conclusions
Especially in children, fibrinolytic therapy cannot be said to be an alternative to surgery in empyema. But in each case of the fibrinopurulent phase empyema that does not respond to closed chest tube drainage, it should be tested. Such treatments increase the success of conservative treatment. VATS or open thoracotomy should continue to be the preferred treatment for complete lung decortication in localized cases.