1. Background
Decreased pulmonary compliance is one of the most common pathologies in newborns’ respiratory diseases, especially in pre-term neonates, due to which continuous distending pressure (CDP) on respiratory airway is considered as standard care in treatment of related diseases (1).
In 1968, Benveniste and Pedersen defined the outline and principles of CDP in neonates. However, in 1971, Gregory et al. focused on the role of CDP as a therapeutic approach in newborns suffering from RDS and operationalized it. In this regard, related research showed that, through mechanisms such as increased functional residual capacity (FRC) leading to improved PaO2 levels, improved pulmonary compliance, strengthened and stabilized airways, strengthened diaphragm performance, avoided alveolar collapse, reduced oxygen pressure gradient at alveolar-arterial levels (A-aDO2), decreased intrapulmonary shunt, reduced obstructive and mixed apnea and protective effect on surfactant, diseases with reduced static compliance can be managed, especially those with reduced dynamic compliance such as RDS (2).
nCPAP (nasal continuous positive airway pressure) as a subcategory of noninvasive-noncycled respiratory support is linked with CDP and is regarded as a basis and the first intervening treatment level in neonates involved with RDS along with surfactant administration. However, a number of challenges could be thought of in institutionalizing this therapeutic approach including maintaining an acceptable pressure level in order to keep FRC in respiratory cycle stable, managing interface leak, preventing imposed WOB in patients, preventing applying levels of trans-pulmonary pressure leading to reducing cardiac output and, as a result, resulting in metabolic acidosis. In more severe cases, it may cause air leak syndrome (3-5).
nCPAP applies air in the injector through two specific categories, namely flow driven CPAP and constant flow CPAP. CPAPs applied through ventilators are usually categorized as constant flow CPAPs. During the last decade, due to the nature of CPAP ventilators (i.e. constant gas flow in a circuit and limited pressure known as constant flow), concerns regarding maintaining optimal levels of CDP flow in application of non-invasive CPAPs have been highlighted (6).
In neonates under mechanical ventilation due to limited compliance, FRC maintenance is considered to be a critical criterion in avoiding patient desaturation since oxygen reserve is limited or may end. Furthermore, challenges such as reduction in static compliance make ventilation more difficult, which results in an increase in WOB. FRC maintenance requires an optimal level of CDP. Unlike invasive ventilation, maintaining distending pressure constant is among the most significant challenges in non-invasive ventilation since gas leakage, which usually accompanies this type of breathing support, leads to decreased pressure. Even in the most accurate sealing, anatomic leakage exists, which may result in failure of this therapeutic approach (non-invasive ventilation) in critical patients (7).
With the introduction of pressure-oriented ventilators in the early 70s followed by development of BIPAP (biphasic positive airway pressure) software programs in the following decades, a promising prospect was shaped to keep a constant pressure in respiratory cycle so as to keep the pressure constant through inspiration and respiration. This led to defining a respiration model called pressure control in mechanical ventilation through development of Autoflow hardware, which can control the pressure in a real time fashion by electronic feedback mechanisms. Today, the practicality of this system is being increasingly emphasized in non-invasive ventilation as well. The aim is to increase or decrease instantaneous gas flow in order to ensure constant pressure through gas leakage compensation, in a way that a body of practical research has been shaped in this area (8).
2. Objectives
However, the capabilities of software applications such as Electronic Feedback Control Valve and Autoflow in leakage compensation in interface, pressure drop compensation or increased CDP pressure compensation in neonates’ inspiration and expiration in clinics seem to be able to manage those challenges against non-invasive CPAPs which are typically observed in clinics including imposed work of breath (WOB) in neonates with diseases that reduce compliance levels or stable pressure level, especially during inspiration, and subsequently, prevent reduction in tidal volumes. This can lead to improved outcomes for patients with the above-mentioned diseases. Being supplied with Servo-i ventilators with these software programs, we decided to assess their clinical performance.
3. Methods
The present study is a prospective randomized clinical trial on 70 neonates weighing about 1000 grams with respiratory distress syndrome admission in NICU in Alzahra Hospital and Shahid Beheshti Hospital associated with Isfahan University of Medical Sciences from August, 2015 to February, 2018.
The inclusion criterion was neonates weighing about 1000 grams with respiratory distress syndrome (Tachypnea, Intercostal retraction, nasal flaring, granting, needing inspired oxygen fraction higher than 21%) and the exclusion criteria were congenital anomaly and perinatal asphyxia (5-minute Apgar score between 0 and 3, umbilical cord pH less than 7 and umbilical cord bicarbonate less than 12 mEq/Lit) (9).
Participants of the study included neonates weighing about 1000 grams selected based on inclusion criteria after related written consent agreements were signed by their parents. Neonates whose first file number digit was an even number were put in the group ‘electronic feedback pressure control constant flow nasal-CPAP’ (PC-nCPAP) and those with an odd first file number digit were grouped as ‘pressure limited constant flow nasal-CPAP’ (PL-nCPAP).
Neonates in PC-nCPAP group were provided with nCPAP respiratory support including Nasal prong Argyle (Covidien, Mansfield, USA) and Servo-i ventilator (Maquet, Solna, Sweden). Servo-i was equipped with a non-invasive ventilation software program and the users selected ‘non-invasive ventilation’ and ‘nCPAP’ prior to activating ventilation. The primary CDP level was set as 6 cmH2O and FiO2 = 30% (10).
The neonates who needed an inhaled oxygen fraction higher than 40% in order to keep oxygen saturation level at 90% - 95% in their right hands received 100 mg/kg of Survanta using INSURE method. Then, if the neonates’ need of inhaled oxygen fraction higher than 40% was kept constant at acceptable levels, Survanta was administered again 6 hours after administration of the previous surfactant dose, which continued maximally for 4 doses. CBG (Capillary Blood Gas) was measured before and after surfactant administration and then continued every 12 hours and, based on that, related mechanical ventilation management alterations were made (11).
Patients with any of the following conditions would be discontinued from non-invasive ventilation and would then undergo intubation and invasive ventilation:
•Despite a CDP of 8 cmH2O and FiO2 ≤ 75%, inability to keep oxygen saturation level at 90% to 95% in their right hands (10)
•Gasometric indices in CBG showing respiratory failure (pH < 7.2 and PCO2 > 65 mmHg) (12)
•More than 3 times of apnea per hour requiring ventilation using a bag and a mask
During respiratory management, in instances when a neonate’s need for fraction of inspired oxygen in levels lower than 50% was kept constant for more than 4 hours, CDP gradually dropped 1 to 2 cmH2O to keep O2Sat at an acceptable range. At CDP = 4 cmH2O and FiO2 < 30%, the neonate was weaned from respiratory support (12).
Neonates in PL-nCPAP group were supported with nCPAP respiratory support using Nasal prong Argyle (Covidien, Mansfield, USA) with the aid of Christina ventilator (Stephan Medizintechnik, Hamburg, Germany). This management was similar to the managerial involvement applied for PC-nCPAP group.
Neonates’ demographic data, duration of non-invasive respiratory support, need for intubation and invasive ventilation, any instances of apnea, need for surfactant administration and the number of additional doses were recorded and monitored. Any instances of pneumothorax and chronic lung diseases were also documented.
RSB (rapid shallow breathing) Index was monitored using the ratio of respiratory frequency to tidal volume (8). Also, WOB of the ventilator was the result of expiratory tidal volume multiplied by dynamic pressure (13). In order to calculate dynamic pressure in PC-nCPAP group, maximum pressure gradient in inspiration phase and 50% of pressure difference between static pressure and positive end-expiratory pressure (PEEP) were used and, for PL-nCPAP group, dynamic pressure equals 50% of maximum pressure in pressure-time signal (14).
The data obtained for 35 neonates in each group was analyzed using SPSS software program version 18 through independent t-test, Pearson coefficient correlation and chi-square test. The confidence level, test power and standard error were 95%, 80% and 0.37, respectively.
4. Results
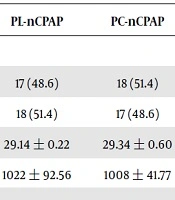
Table 1 shows demographic indices for both groups. The average gestational age for PL-nCPAP and PC-nCPAP groups showed no significant difference between the two groups (P value = 0.48). There was no significant difference between the two groups in terms of their average weights (P value = 0.61). Antenatal steroid administration in PL-nCPAP was not significantly different from that of PC-nCPAP (P value = 0.54). Moreover, no significant difference was observed between the two groups regarding route of delivery (P value = 0.19). Concerning preterm rupture of membranes for 18 hours or more, no significant difference was observed in the two groups studied (P value = 0.11). The mean Apgar scores for the neonates in PL-nCPAP and PC-nCPAP groups in minutes 1 and 5 showed no significant difference between the two groups of this study.
| PL-nCPAP | PC-nCPAP | P Value | |
|---|---|---|---|
| Sex | |||
| Male | 17 (48.6) | 18 (51.4) | 0.998 |
| Female | 18 (51.4) | 17 (48.6) | |
| Gestational age, wk | 29.14 ± 0.22 | 29.34 ± 0.60 | 0.481 |
| Weight, g | 1022 ± 92.56 | 1008 ± 41.77 | 0.612 |
| Apgar | |||
| 1 minute | 5.7 ± 0.57 | 5.69 ± 0.47 | 0.529 |
| 5 minutes | 7.41 ± 0.66 | 7.43 ± 0.50 | 0.595 |
| Steroid administration | 31 (88.6) | 29 (82.8) | 0.54 |
| ROM ≥ 18 hrs | 18 (51.4) | 16 (45.7) | 0.11 |
| Rout of delivery | |||
| NVD | 14 (40) | 8 (22.9) | 0.197 |
| C/S | 21 (60) | 27 (77.1) |
Abbreviations: C/S, caesarean section; NVD, normal vaginal delivery; PC-nCPAP, pressure control-nasal continuous positive airway pressure; PL-nCPAP, pressure limited-nasal continuous positive airway pressure; ROM, rupture of membrane; SD, standard deviation; wk, week
aValues are expressed as No. (%) or mean ± SD.
In Table 2, the respiratory and clinical outcomes in both groups are compared. The average non-invasive treatment duration comparison in both groups showed no significant difference between the two groups (P value = 0.87). The two groups showed no significant difference in their need for invasive ventilation (P value = 0.45). The average need for surfactant administration and additional doses was significantly lower in PC-nCPAP group (P value = 0.003). The average WOB of the ventilator was significantly higher in PC-nCPAP group compared with that of PL-nCPAP group (P value < 0.001). The average RSB index was significantly higher in PL-nCPAP group (P value < 0.001). Furthermore, the comparison of the mean of chronic lung diseases, intraventricular hemorrhage, pneumothorax, and the number of deaths showed no significant difference between both groups.
| PL-nCPAP | PC-nCPAP | P Value | |
|---|---|---|---|
| Length of non-invasive support, ha | 144 ± 25.30 | 147.43 ± 39.40 | 0.873 |
| RSB indexa | 22.212 ± 3.7 | 10.445 ± 2.1 | < 0.001 |
| Mechanical ventilation | 14 | 10 | 0.450 |
| CLD | 18 | 18 | 0.785 |
| IVH | |||
| Grade 1 | 5 | 4 | 0.974 |
| Grade 2 | 18 | 16 | |
| Grade 3 | 3 | 7 | |
| Grade 4 | 2 | 0 | |
| Pneumothorax | 7 | 5 | 0.749 |
| Surfactant administration | |||
| Totally 1 dose | 2 | 11 | 0.003 |
| Totally 2 doses | 7 | 11 | |
| Totally 3 doses | 16 | 8 | |
| Totally 4 doses | 8 | 1 | |
| V-WOB, Ja | 0.0009 ± 0.00015 | 0.004 ± 0.001 | < 0.001 |
| Dead | 8 | 6 | 0.766 |
Abbreviations: CLD, Chronic lung disease; IVH, Intra-Ventricular Hemorrhage; PC-nCPAP, Pressure Control-nasal Continuous Positive Airway Pressure; PL-nCPAP, Pressure Limited-nasal Continuous Positive Airway Pressure; RSB, Rapid shallow breathing; SD, Standard deviation; V-WOB, Ventilator Work of Breathing
aValues are expressed as mean ± SD.
5. Discussion
A number of studies have been conducted regarding the performance of respiratory machines in order to assess their capability to compensate for gas leakage in non-invasive respiratory support in terms of software and hardware and thus maintaining and stabilizing the pressure applied on airways (in respiratory cycle), which have been accompanied by respiratory simulation. Anatomic leakage is inevitable in non-invasive ventilation. However, in terms of interface techniques, gas leakage may increase the magnitude of the leakage.
Oto et al. (15) conducted a study using non-invasive ventilation of a respiratory simulator (Pediatric Lung Simulator Hudson, Temecula, California) in which they used a number of ventilators including CareFusion Avea, Maquet Servo-I, Drager V500, Covidien PB840, Respironics V60, GE Healthcare/Engstrom Carestation and Hamilton C3. Given CDP = 5 cm/H2O and CDP = 10 cm/H2O in a scheduled interface, gas leakages in levels of 2 - 3 L/min, 5 - 6 L/min, 9 - 10 L/min and 19 - 20 L/min were designed. The capability of each respirator to compensate for gas leakage and keep the pressure stable was assessed. Results of this study showed that Covidien PB840 and Hamilton C3 were significantly capable of showing minimal differences through the defined pressure for the airways in the respiratory cycle, both in low and high pressures and in all levels of gas leakage (15).
In another study designed by Drevhammer et al. (16), a respiratory simulator (ASL, 5000, IngMar Medical, Pittsburg, PA) capable of showing a variety of pulmonary volumes at different weights was used. The defined weights for this simulator included 1.3 and 3.4 kg. The study aimed at investigating the capability to keep CDP constant while applying nCPAP and also investigating the imposed WOB in simulated patients, which, of course, is observed during non-invasive CDP. The study was conducted using seven ventilators including AVEA (CareFusion, Yoba Linda, CA), VN500 (Drager Medical, Lubeck, Germany), Engstrom Carestation (GE Healthcare, Little Chalfont, UK), Evita XL (Drager Medical), Fabian (ACUTRONIC, Hirzel, Switzerland), Leoni Plus (Heinen Lowenstein Bad Ems, Germany) and Servo-I (Maquet, Solna, Sweden). CDP was defined in a non-invasive respiratory pattern in pressures of 3, 4, 5, 6, 7, 8 and 9 cmH2O. Monitoring results showed that Fabian was significantly efficient in keeping the pressure constant during respiratory cycle while Heinen showed the highest level of pressure inconsistency. Moreover, the imposed WOB was significantly low as measured in Fabian and Evita XL (16).
As mentioned above, Servo-i was one of the basic respirators in these studies. In 2006, a company called Maquet, with the aid of Getinge group, developed software compatible with hardware controlling expiratory value and gas flow speed while Siemens designed the new generation of pneumotachograph sensors capable of monitoring the volume of respiratory gases using ultrasonic waves through the process of electronic feedback. Servo-i was equipped with this hardware in order to eliminate structural weaknesses in non-invasive ventilators, especially applying CDP with the lowest changes in pressure levels in a respiratory cycle. The designers claim that the final product can compensate for leakage up to 25 L/min and prevent pressure loss effectively in a respiratory cycle (17).
Following the introduction of a mechanism called electronic feedback control value in Servo-i ventilators, a number of studies were conducted concerning the system capabilities compared with other CPAP machines which used different mechanisms. Shannon et al. (2010) studied imposed respiratory resistance and its effects on tidal volume. A neonatal respiratory simulator (ASL 5000 IngMar Medical, Pittsburge, Pennsylvania) was used in this study. The simulator was programmed so that it could demonstrate a neonate weighing about 1000 grams involved in RDS with compliance equal to 0.5 mL/cmH2O. Tidal volumes experienced a gradual increase during applying nCPAP (3 mL, 6 mL, 9 mL, and 12 mL). The studied CPAP machines included Servo-i (Maquet, Solna, Sweden), Bubble CPAP (Fisher & Paykel, Auckland, New Zealand), Airlife (Cardinal Health, Dublin, OH, USA) and Arabella (Hamilton Medical, Bonaduz, Switzerland). Among these, Servo-i was equipped with an electronic feedback system in order to compensate for gas leakage up to 25 L/min. Pressure loss during inspiration was at a minimum level in Airlife, which was designed based on flow opposition. This was more observable in higher tidal volumes and showed to be statistically meaningful compared with other CPAP machines (P < 0.001). Difference in pre-set and measured tidal volumes during inspiration was significantly minimum (P < 0.001) in Servo-i compared with other CPAP machines (18).
In the present study, which seems to be the only study conducted investigating the software programs capable of compensating for high levels of gas leakage during non-invasive respiratory support at the bedside, a number of other indices were also studied, which can be regarded as the ground for further studies in this regard.
5.1. Conclusions
In PC-nCPAP group, WOB of ventilator was significantly higher than that of PL-nCPAP group. Based on significantly higher ventilator’s WOB, significantly lower RSB and significantly lower surfactant administration in PC-nCPAP group compared with PL-nCPAP, it can be concluded that WOB in neonates in PC-nCPAP group was not as much as that of PL-nCPAP group, making the neonates in the former group experience more stable conditions. The present study can pave the way toward doing more research in this area since the future perspective seems to be promising.
5.2. Limitations
The present study may render short of measuring the actual neonates’ WOB using a plethysmography machine. However, we could not find a related study on neonates in this regard.